
东莞某医疗器械有限公司成立于2016年,坐落于东莞长安。专注于医疗产品设计及制造,拥有专业且完整的开发产品和生产经验;产品主要出口英国和欧盟,其合作伙伴皆为全球知名医疗器械品牌。瑞恩尼本次助力企业获得一次性吸洗套件CE MDR(同时也是瑞恩尼助力客户获得的第19张)证书,也祝愿企业财源广进!!
51
深圳市某科技有限公司创立于2012年,是一家致力于家用医疗产品研发、制造、销售及售后服务为一体的高新技术企业。产品涵盖雾化器(压缩式、压电网式、直流压缩式),防褥疮气床垫,而氧仪、胎心仪、血压计等,业务遍及全球150多个国家,服务年均超过2000000个家庭。企业秉承用心缔造健康,致力于提升雾化方式的多元化,让家用医疗产品和现代医学科技惠及全球千家万户为理念,以尊重,包容,正直,高效,创新,执着为核心价值观,整合优势资源,以创新为发展之源,以科技为制胜之本,力争为每个消费者提供更专业,更先进,更安全的医疗产品。本次瑞恩尼助力企业获制氧机欧盟CE MDR证书(该证也是瑞恩尼助力企业获得的第18张),祝企业生意兴隆!
69
深圳某科技有限公司成立于2015年,经营范围包括一般经营项目:母婴用品制造;母婴用品销售;化妆品零售;家居用品制造;家居用品销售;医护人员防护用品生产(Ⅰ类医疗器械);第一类医疗器械销售;国内贸易代理;互联网销售(除销售需要许可的商品);照相机及器材销售;照相机及器材制造;家用电器研发;家用电器销售;电子产品销售;通讯设备销售。模具制造;模具销售;图文设计制作;专业设计服务;工业设计服务;工业工程设计服务。(除依法须经批准的项目外,凭营业执照依法自主开展经营活动)许可经营项目:第二类医疗器械销售;货物进出口;技术进出口;化妆品生产;医护人员防护用品生产(Ⅱ类医疗器械)。(依法须经批准的项目,经相关部门批准后方可开展经营活动,具体经营项目以相关部门批准文件或许可证件为准)本次瑞恩尼助力企业完成加拿大、日本、美国MDSAP体系的建立并成功获得MDSAP认证证书。
63
Dongguan Electronic Technology Co., Ltd. is a professional manufacturer specializing in medical equipment products such as infrared forehead thermometers, infrared non-contact thermometers, electronic thermometers, electronic blood pressure monitors, nebulizers, etc. The enterprise integrates solution development, product design, finished product manufacturing, and market sales. The company has a manufacturing base of over 10000 square meters, equipped with modern production lines and more than 300 well-trained employees. It is proud that the company has a dynamic R&D technology team. They have over 10 years of experience in the electronic healthcare industry, always standing at the forefront of medical electronics technology and tirelessly serving the general public with technological innovation. Moreover, the enterprise always attaches great importance to the control of production, quality, and logistics links, striving for products that are cost-effective. The above advantages not only make the company's own brand well-known, but also provide effective guarantees for customized services to customers. The enterprise has passed ISO13485 certification and obtained a medical device manufacturing enterprise license. And it has multiple CE MDR certificates and FDA 510k certifications for its products.This time, Rui Enni helped the company obtain CE MDR certification for multiple products. We wish the company's products great success and long-lasting sales!
251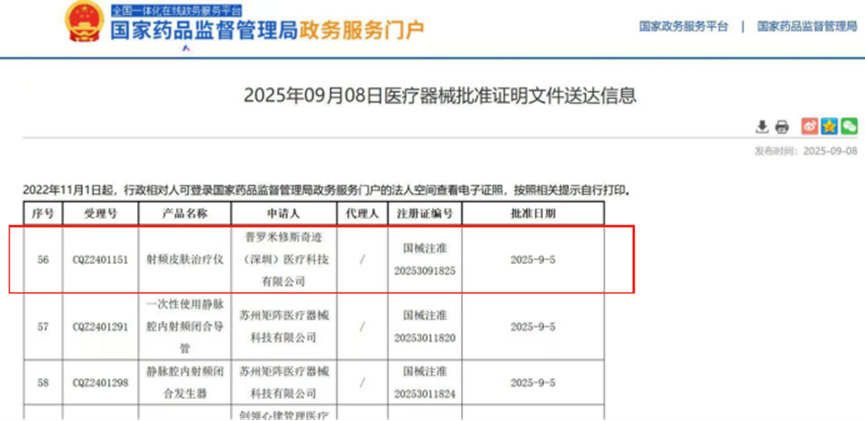
Good news: Guangzhou Zhuyitong has successfully approved the launch of another household radiofrequency skin treatment device On September 5, 2025, the third type of medical device "radiofrequency skin therapy device" developed and produced by Prometheus Miracle (Shenzhen) Medical Technology Co., Ltd. has been officially approved by the National Medical Products Administration, with registration certificate number: National Medical Device Approval No. 20253091825. This project is fully managed by our company Guangzhou Zhuyitong Pharmaceutical Consulting Co., Ltd. (hereinafter referred to as "Zhuyitong") throughout the entire clinical trial process, from project initiation to completion, all independently managed by Zhuyitong. This project is the first approved handheld three function (RF, LED, and EMS) RF skin therapy device. This approval marks the second certificate of the radiofrequency skin therapy device for clinical trial services provided by Guangzhou Zhuyitong. This is not only a recognition of the customer's product capabilities, but also an indirect recognition of our team's hard work and professional abilities! The following is the experience sharing of Zhuyitong on this clinical trial: 1、 Project BackgroundWith the expansion of the medical beauty market, relevant departments in China have also standardized and regulated the medical beauty industry to ensure its healthy development. On March 30, 2022, the National Medical Products Administration issued a notice (No. 30 of 2022) on adjusting some contents of the "Classification Catalogue of Medical Devices", which includes adjustments to the contents of the "Classification Catalogue of Medical Devices" for 27 categories of medical devices. According to the relevant requirements of this announcement, radiofrequency beauty equipment is clearly managed as a Class III medical device. For the radio-frequency skin therapeutic instrument products in the 09-07-02 radio-frequency treatment (non ablation) equipment involved in the adjustment in the annex, from the date of the announcement, it is necessary to apply for registration in accordance with the Administrative Measures for the Registration and Filing of Medical Devices (Order No. 47 of the State Market Supervision and Administration). Starting from April 1, 2024, radiofrequency skin therapy devices cannot be produced, imported, or sold without obtaining a medical device registration certificate in accordance with the law. Starting from April 1, 2024, enterprises that have not obtained a medical device production and operation license (filing) are not allowed to engage in the production and sale of related products.In April 2023, the Medical Device Technical Evaluation Center of the National Medical Products Administration issued the "Guiding Principles for Registration and Review of Radiofrequency Beauty Devices". The clinical evaluation requirements for radiofrequency beauty devices in the guiding principles should refer to the recommended path for clinical evaluation of products related to "Physical Therapy Devices" in subdirectories 09 of the "Classification Catalogue of Medical Devices". Recommend conducting relevant evaluations through clinical trial pathways. At this critical juncture of regulatory upgrading in the industry, Zhuyitong has been entrusted by Prometheus Miracle (Shenzhen) Medical Technology Co., Ltd. with full responsibility for the clinical trial project of its radiofrequency skin therapy device, based on its professional reputation. This fully demonstrates the applicant's deep trust in Zhuyitong.2、 Project milestonesThe clinical trial of this project lasted approximately one year from the finalization of the protocol design to the release of the trial summary report. From the initial stage of the project's clinical trial plan design, the team collaborated with multiple medical beauty clinical experts and data statisticians to conduct multiple rounds of argumentation, repeatedly optimizing inclusion/exclusion criteria, evaluation indicators, and sample size calculation models to ensure that the plan not only meets the requirements of the "Guidelines for Registration and Review of Radiofrequency Beauty Equipment", but also has clinical operability; Screening and conducting research at the center, inspecting the medical beauty departments of multiple tertiary hospitals across the country, and ultimately determining two clinical centers that meet the requirements; In the ethical approval stage, actively cooperate with the hospital's ethical requirements to submit materials, complete the ethical meeting of the team leader unit within one month and obtain approval; And the subsequent center launch meeting organization, full cycle management of subjects (with a cumulative follow-up dropout rate of only 1.4%), third-party inspection cooperation (no major quality issues), data statistical analysis, until the writing of the summary report and submission of registration materials, each link embodies the hard work of the Zhuyitong team.During the project promotion process, as the handheld radiofrequency skin therapy device was first placed under medical device supervision, there was neither consensus among clinical experts nor clinical precedents to refer to in China. During the process of scheme design and review, relevant parties actively communicated and cooperated, successfully completing all clinical trials and review Q&A, and ultimately obtaining approval.3、 Project milestonesAt the end of November 2024, the project will undergo on-site inspection by the National Medical Products Administration. Zhuyitong dispatched all trial participants to provide on-site assistance throughout the entire process, offering professional technical support and process coordination to ensure efficient and smooth verification work, further confirming the team's professional ability and responsible attitude. Figure 1: Welcome Meeting for On site Verification by Relevant National Regulatory Authorities Figure 2: Medical Assistance Team Members Assist in On site VerificationThe passing of GCP inspection marks that the clinical trial process of Zhuyitong conforms to the principles of standardization and science, and can withstand the review of relevant parties and regulators. This is an example that proves the standardization of clinical management in Zhuyitong, and it is also a good start. It is also a reference example for more clinical trial projects in the future, and a sign of progress and continuous improvement in the future.3、 ConclusionThe successful approval of a medical device relies on the collaborative efforts of the sponsor, clinical research unit, CRO, and SMO teams. Zhuyitong is fortunate to be a key participant, providing professional services to sponsors and clinical research units. In the future, it will continue to leverage the professional advantages of CRO clinical services and registration to support the development of the medical beauty industry!In the journey of innovative medical devices from the laboratory to the market, we are well aware of the challenges that enterprises face: complex regulations, rigorous clinical validation, and lengthy approval cycles. As a deep vertical medical device CRO service organization, our company is willing to become your most reliable strategic partner, providing full cycle solutions from research and development planning to successful registration.Forward planning and risk reduction: In the early stages of product development, we intervene to provide registration strategy consulting and clinical pathway planning to clarify your direction and avoid fatal defects in the technical review stage.Efficient execution and quality assurance: We have a strong team of experts and execution network, providing one-stop management services for clinical trials, including protocol design, center screening, ethical approval, monitoring and inspection, data management and statistics, to ensure compliance in the trial process and solid data.Successful application, perfect conclusion: We are proficient in registration regulations and can efficiently write and integrate key application materials such as clinical evaluation reports (CERs) for you. We also provide professional guidance for examination preparation, greatly improving the success rate of registration.We not only deliver projects, but also deliver success and peace of mind. Based on the successful cases of previous projects, we promise to provide the highest professional standards and the most responsible attitude to escort your innovative products throughout the process, helping them to be launched as soon as possible, benefiting patients, and winning commercial success.At present, the clinical trial projects being carried out by Zhuyitong include IPL hair removal device, hearing aids, HIFU products, blood oxygen, etc. Zhuyitong has a professional and experienced clinical trial team, including PM, clinical medicine, statistics, CRA, and SMO.Contact us immediately for exclusive listing solutions!
440
Shenzhen Medical Technology Co., Ltd. was founded on January 14, 2020. The main business scope includes general business projects such as sales of Class I medical devices, development of electronic medical equipment, and technology for external defibrillators; Import and export of goods or technology. The licensed business items are: the production and operation of Class II and III medical devices has 1-20 people, and the nature of the enterprise is limited liability company, which belongs to Shenzhen mobile Internet industry.Rui Enni provides domestic Class III GMP system guidance for enterprises, from GMP system introduction, transformation, implementation to on-site verification and evaluation. And successfully assisted the enterprise in passing the on-site inspection of the national bureau. The company has obtained the "Semi automatic Defibrillator Medical Device Registration Certificate" issued by the National Bureau. Congratulations to the company for obtaining the certification, and wish the product great sales!!
357Phone
0755-27391220
020-82513196

WeChat customer service

Mini Program
reanny@reanny.com