
Development history of hearing aidsHearing aids are important auditory aids that help people with hearing impairments reintegrate into society and enjoy wonderful sounds and rich environmental sounds. The origin of hearing aids can be traced back to the 17th century. After centuries of changes and innovations, hearing aids have evolved from mechanical devices to electronic devices and then to digital devices. Technological advancements have made hearing aids smaller and more in line with human needs, resulting in improved performance and a better user experience.Conventional hearing aids generally require a professional doctor to test the patient's hearing impairment, and then configure them professionally based on the test results to provide better hearing compensation to the patient. Until 2021, the United States approved self fitting hearing aids that can be sold and used directly to consumers without the assistance of hearing professionals, bringing about a significant change in the convenience and experience of hearing aid use. Users no longer need to go to the hospital or seek professional audiology experts. They can purchase a hearing aid and use the accompanying app to test their hearing loss and configure suitable hearing aids to compensate for hearing loss.※ Classification of hearing aids in the United StatesThe United States has divided hearing aids into 19 product codes and 9 regulation numbers, of which 7 codes require 510 (k) registration, while other hearing aids are exempt from 510 (k) registration. For a more detailed description of the types of hearing aids, please refer to our other article titled "Hearing Aids and Personal Sound Reinforcement Products: Notes"( https://mp.weixin.qq.com/s/BMpr5vNnc6cVNoVHs_HFDA ). With the advancement of technology and the emergence of self fitting hearing aids, in 2022, the US Food and Drug Administration added the product code "QUH" for over-the-counter self fitting hearing aids on the basis of the original product code "QDD", distinguishing prescription and over-the-counter self fitting hearing aids.※ Registration processIn this self fitting hearing aid application, we went through the transition of product code from "QDD" to "QUH". The process from submitting no clinical trials and usability validation to issuing additional clinical trials and usability validation. After receiving the FDA's request for clinical trials and usability, I started preparing the clinical trial and usability validation plan, and submitted Q-Sub to discuss the feasibility of the plan with the FDA. After unremitting efforts and communication, we finally determined the clinical trial and usability plan.The clinical trial was conducted by Ruieni Group's Zhuyitong Company in two hospitals within China; SMO was completed by Borussia of Ruini Group; The usability study was planned, statistically analyzed, and summarized by Ruini Consulting, and the 510 (k) registration process from submission to submission was completed by Ruini Consulting. Thanks to the active efforts of Ruini and the client, the submission of the supplementary documents was finally completed before the deadline for submission.It took more than 5 months from submission to certification, and finally completed registration at the end of September, becoming a Chinese [sensitive word] OTC self fitting hearing aid approved by the US FDA.Experience sharingAs the service provider in China who obtained this case, Ruini would like to share its experience in OTC self fitting hearing aid registration.1. Non prescription hearing aids can be used by non professionals, who can directly purchase, use, or operate them, thus requiring higher safety requirements than prescription hearing aids. Among them, the maximum output limit for loud sound should not exceed 117dB at any frequency; the material of the earplug must be undamaged and the depth of the sensitive word must be kept at least 10mm away from the eardrum.2. When measuring the electroacoustic performance of an acoustic coupler, a 2cm3 acoustic coupler should be selected. Only when the 2cm3 acoustic coupler is incompatible with the hearing aid, other scientifically effective and technically equivalent acoustic couplers should be considered, and the basic principles of using alternative acoustic couplers must be recorded.3. Usability verification should include all expected usage environments, characteristics of the user population, and the proportion of experienced personnel. The subjects should perform the test under adverse conditions and collect any usage error information or data such as decision-making errors, operational errors, interpretation errors, etc., and conduct a summary analysis to confirm whether these errors affect the safety and effectiveness of the product.4. The FDA may accept clinical trials conducted outside the United States, but it is necessary to ensure that the clinical data is sufficient, truthful, ethical, and scientifically effective. This clinical trial is a randomized, crossover, single blind, non inferiority multicenter clinical trial. The experiment is divided into two stages: laboratory stage and on-site cross wearing stage, and effectiveness (including primary and secondary indicators) and safety analysis are conducted for each stage. ※ ConclusionThe approval of 510K OTC self fitting hearing aids is a credit and honor that belongs to the customer, colleagues from Ruini Group, clinical researchers, and statistical experts. It is the result of the joint efforts of all personnel working together!!!
1452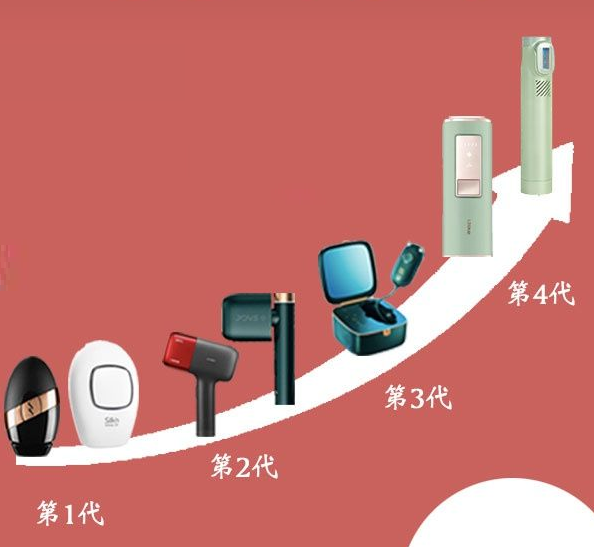
Intense Pulsed Light (IPL)Intense pulsed light (IPL) is a non laser light source composed of a single or multiple pulse sequence of incoherent light, characterized by a wide spectral range and high energy density. Short wavelength light can be filtered out using filters or coatings. For example, the 560 treatment head filter retains the spectrum within the wavelength range of 560nm to 1200nm, except for the band shorter than 560nm. The light source of the strong pulse light device is a xenon lamp, and its basic working principle is that a trigger applies high voltage to xenon gas to trigger xenon ionization. After being charged for a relatively long time through an energy storage capacitor, it discharges in a very short time, causing avalanche ionization of xenon gas inside the lamp tube. Xenon gas converts and releases the charged energy in the form of high-intensity light radiation, and this discharge process is a light pulse.Using strong pulsed light with a certain penetration depth and good absorption ability in melanin, the hair follicles and hair shafts irradiated by light absorb a large amount of light energy, which is converted into heat energy and transmitted to the end of the hair follicles through the protein inside the hair, causing the entire hair to be heated. The protein and cells undergo coagulative necrosis, ultimately leading to the loss of the entire hair due to the necrosis of growing cells.In the past, common hair removal methods in family environments included hair removal cream, wax paper, and hair scraper, which belonged to chemical or physical hair removal. Hair removal wax paper uses brute force to remove hair, which not only causes strong pain, but also easily leads to folliculitis; The hair removal knife only removes the surface hair, so frequent scraping is needed to maintain it, and there is a risk of scratching; Hair removal cream belongs to chemical hair removal, which uses chemical substances to make hair fragile and prone to breakage, achieving the effect of hair removal. It is easy to cause skin allergies, has a short maintenance time, and has high long-term and large-scale use costs.At present, the common hair removal equipment in hospitals or beauty institutions is generally a large multifunctional strong pulse light therapy device with a foot pedal. With the development of technology, IPL strong pulse light technology can be used in home environments and medical beauty institutions, and the equipment has become increasingly compact and portable through upgrades and iterations. Recently, the strong pulse light therapy device assisted by Shenzhen Ruienni submitted an application according to the registrant system and was awarded one of the first batch of medical device registration certificates approved by the Guangdong Provincial Drug Administration for clinical evaluation (registration certificate number: Yuexizhun 20232090285).During the registration process, the evaluation center focused on some key points with strong professional skills, such as the cut-off wavelength of the filter, the number of pulses, and the method of energy adjustment.During the rectification process, a technical evaluation expert review meeting was held to address the differences in pulse energy and energy density between the proposed registered product and the comparison devices of the same variety. We have made sufficient preparations in advance before this meeting. During the meeting, we provided a comprehensive and detailed explanation and examples of the product's technical principles, structural materials, functions, safety and effectiveness related to clinical evaluation. The results of the meeting were unanimously recognized by the expert group.Ruini lives up to the customer's trust! Within just over a month of submitting supplementary materials after the expert review meeting, we successfully obtained the registration certificate for the "Intense Pulse Light Therapy Device" issued by the Guangdong Provincial Drug Administration for clinical evaluation approval.Here, we would like to share some points that need to be noted during the clinical evaluation and review process of selecting the same variety for NMPA registration:When selecting the same variety for comparative analysis and clinical evaluation, it is necessary to focus on the technical key points of implementing various functions of the product, such as the wavelength range of terminal output pulse light, pulse energy, and energy density.1. Wavelength range: In the wavelength range of most registered and marketed devices, the long wavelength can reach 1200nm, while some products have a long wavelength of only 950nm or even shorter, or longer than 1200nm. When submitting clinical evaluation terminals for comparative analysis of the same product, it is necessary to explain the safety and effectiveness of the product from both theoretical and clinical perspectives.2. Pulse energy: Excessive pulse energy can cause varying degrees of adverse events. In comparative analysis, if the pulse energy of the device to be declared is higher than that of the equipment compared with the same variety, it is necessary to explain in detail the method of controlling the use of energy in the product, as well as the numerical changes in pulse energy and energy density to ensure the safe use of the equipment.
881
The IPL hair removal device generally consists of a host, a treatment head, and a filter. It is an over-the-counter device used to remove excess hair from adults and is suitable for use at home or in medical institutions. Regulated in the United States according to Medical Device Class II, product code: OHT.Figure 1 IPL hair removal device product imageThe IPL hair removal device utilizes the blackness of hair to absorb intense pulsed light (IPL), converting energy into heat, causing protein coagulation and necrosis, and promoting hair loss.In 1975, Thomas Fitzpatrick, an American dermatologist, classified the skin of people of all races into six categories, namely Type I to VI. This classification is based on the original color of the skin (non illuminated area), the color of the eyes and hair, and the skin's response to light. The IPL hair removal device is suitable for adults with Fitzpatrick I-IV skin types. Users with skin types V and VI have higher levels of melanin in their skin and are more likely to experience adverse events after use. Therefore, it is not recommended for people with skin types V and VI.Note: The Fitzpatrick classification information comes from Skin typing: Fitzpatrick grading and others, Vishal Gupta, Vinod Kumar Sharma; Clin Dermatol. 2019 Sep-Oct; 37(5):430-436.The IPL hair removal device applied for this time has a cooling effect, and the key component for cooling is the cooling plate, which cools based on the Peltier effect. When current flows through the contact point formed by two different conductors, electrons flow from the low-energy P-type material to the high-energy N-type material. Electrons jump from the low-energy level to the high-energy level, which is manifested as electron heat absorption, forming a cold surface (the cold surface of the cooling plate), and then in the process of hair removal, contact cooling is carried out through the skin of the contact area.The common hair removal devices on the market are Ice Point and Sapphire Hair Removal Devices, both of which achieve cooling effect through cooling fins. The difference lies in the difference in the cooling fins, with the former being aluminum alloy and the latter being sapphire, and the cooling fins being the same; Secondly, sapphire can reduce energy leakage, have fast light output speed, and improve hair removal speed.The main regulations that the IPL hair removal device application process must comply with are shown in the table below:In order to effectively pass the audit and minimize the risk of issuing supplements as much as possible, we utilized our rich FDA work experience in the early stages of accepting customer commissions to take preventive measures for customer product testing, instruction manual specifications, etc. Ryan Ni submitted a complete set of application materials to the FDA on August 29, 2022, and the entire review process went smoothly. During the interactive review, he provided explanations and clarifications on several unclear areas for the reviewers, but did not raise any additional issues. Received an official approval letter from the FDA on November 17, 2022. The entire submission to approval process takes no more than 3 months. (K-number: K222598) During the process of preparing FDA 510 (K) application materials, special attention should be paid to:1) Selection and comparison instructions for substantially equivalent devices. The declared device and the compared device should have the same expected use, applicable population, applicable skin color, applicable hair color, wavelength, energy density, pulse duration, output energy, service life and other performance parameters. When the performance parameters are different, the differences should be reasonably explained to ensure that they will not cause safety and effectiveness issues. If necessary, relevant testing data should be provided for demonstration. When selecting a comparison instrument, choose one. When there are multiple comparison instruments, choose the primary one.2) Household medical devices have strict requirements for product labels and instructions, which must be complete and comply with regulatory requirements. The content should be presented in a visual way such as charts, and the grammar should conform to the local language habits in the United States. Therefore, it is recommended to have personnel familiar with the local language in the United States review the grammar of the instructions before application.
791
Congratulations to Ruini for obtaining FDA 510 (K) approval for the electric nasal cannula in the United States within 2 months, once again breaking the record for fast FDA 510 (K) approval in the United States!Electric nasal suction devices generally consist of a main body and suction components, and are a product used to remove nasal secretions and mucus from children. Regulate according to medical devices in the United States.Ryan Ni was commissioned by a client to handle their company's electric nose wash FDA510 (K) in April 2022. After obtaining the product input information, complete the FDA 510 (K) application materials and submit the complete set of application materials to the FDA official on August 23, 2022. The entire review process was very smooth, and during the interactive review, explanations were given for several mistakes and areas that the auditor was unclear about, but no other issues were raised. Received an official approval letter from the FDA on October 21, 2022. The entire submission to approval process takes no more than 2 months.Ruini lives up to the customer's trust! Accurately grasping product features and mastering FDA regulatory requirements are the conditions for quickly obtaining FDA 510 (K) approval. The concept of professionalism, focus, and dedication is the key to achieving certification success.
621
Recently, the blood glucose meter and blood glucose test paper (glucose dehydrogenase method) products of Shenzhen Aole Medical Equipment (Shenzhen) Co., Ltd. have been awarded the NMPA registration certificate for medical devices by the Guangdong Provincial Medical Products Administration with the assistance of Shenzhen Ruini, adding new members to blood glucose monitoring!Here, Ruini shares some points that need to be noted during the NMPA registration review process for blood glucose meters and blood glucose test strips:
639
Background IntroductionWith the continuous improvement of the living standards of the domestic people, their pursuit of beauty is unprecedentedly high. Based on market demand, a large number of household beauty devices have emerged, such as IPL hair removal devices, phototherapy products, radiofrequency beauty devices, Therma, etc. Based on the changing demand in this market, in order to reasonably control the risks of beauty devices, the Food and Drug Administration is gradually referring to the regulatory model of the US FDA and incorporating beauty devices as medical beauty devices into the scope of medical devices for management in batches.In March 2022, the bureau officially issued a notice requiring the inclusion of radiofrequency beauty devices in medical device supervision and their entry into the market under medical device licensing from April 1, 2024. In April 2022, the bureau issued a notice soliciting opinions on the "Guiding Principles for Classification and Definition of Radiofrequency Beauty Products" (draft for comments). RF beauty devices are classified as follows: In July 2022, the bureau officially proposed classification and definition opinions for radiofrequency beauty products: Medical beauty is a new opportunity and trend in the development of medical devices. Based on this, Guangdong Province has issued relevant opinions on supporting the development of the medical beauty industry - the "Letter from the Office of the Guangdong Provincial Health Commission on Soliciting Opinions on Medical Beauty Surgery Projects and Their Graded Management Catalogue in Our Province" (hereinafter referred to as the "Draft for Soliciting Opinions").With China's economic growth and consumption upgrading, the medical beauty industry in China has developed rapidly, and its market size has jumped to second place. The industry has great development space and fierce market competition. The market size of China's medical beauty market has increased from 99.3 billion yuan in 2017 to 1891 billion yuan in 2021, with a compound annual growth rate of 17.5%. The growth rate of China's medical beauty market is much higher than the total market, and it is expected that the market size of China's medical beauty market will reach 226.7 billion yuan in 2022. Section 2: Strong AllianceRui Enni Consulting has been working in the medical device consulting industry for 9 years and has rich experience in domestic and international registration of medical devices. At present, there are 20 full-time consultants with complete project service capabilities (including design review for domestic and international markets, factory specifications, registration agency, system counseling, clinical trials, production licensing, education and training, and long-term consulting services).The customer registered for the NMPA radiofrequency skin therapy device was established in 2003 and is one of the earliest high-tech enterprises in China focused on the medical beauty industry. The enterprise has numerous high-quality talents with strong capabilities in design and development, quality management, and manufacturing, providing ODM and OEM services for top domestic and foreign brands.In the context of medical beauty enterprises, the client company has decided to register Class III radiofrequency skin therapy devices based on their own development needs. After multiple rounds of negotiations and discussions with various consulting firms, the client ultimately chose Rui Enni Consulting. This registration project is an important "iconic Class III medical device registration project" for Ruienni in the field of medical beauty devices. Ruienni is determined to make this RF project a golden model for enterprise services. It marks an important milestone in the development of Ruini in 2022; It also marks Ryan's active progress towards higher, faster, and more professional directions. This project has significant strategic importance for Rainey. At the beginning of the project kickoff meeting, the client's project representative delivered a warm speech. During the process, the customer representative explained the strategic significance of this registration project from the perspective of the enterprise itself, as well as the challenges and opportunities that the enterprise will soon face.Mr. Wang, the founder of Ruini, was also invited to deliver a speech. The project was summarized from various aspects such as the difficulty of product registration, registration plan, and project service team, and received warm applause from the client.At the end of the meeting, the founder of Ruini (left in the picture below) and the general manager of the client company (right in the picture below) signed the "NMPA Registration Agency Consulting Contract for RF Skin Treatment Equipment", and the project was officially launched. Section 3: OutlookAfter the project is signed, the next step will be for all members of the Ruini project team to participate in the service process. I believe that with mutual trust, sincere cooperation, and efforts from both parties, we can obtain the Class III product registration certificate and production license within the target plan.When we receive good news in the future, we will publish another article to report it to everyone!!!
607Phone
0755-27391220
020-82513196

WeChat customer service

Mini Program
reanny@reanny.com