
Shenzhen Technology Co., Ltd., headquartered in Nanshan District, Shenzhen, is an intelligent hardware company driven by the integration of technology and fashion products. The company's main business is the research and development of intelligent hardware and the production of high-end personal care products. Its brands focus on the research and manufacturing of technology beauty instruments, covering categories such as hair removal devices and photon beauty devices.This time, Rui Enni assisted the company in obtaining the Saudi SFDA certification for the IPL hair removal device, opening up the market for the product in Saudi Arabia. Wishing the company a great success in product sales!
468
Shenzhen XX Medical Equipment Co., Ltd. was established in 2010. It is a specialized enterprise that integrates the research and development, design, production, manufacturing, sales, and service of compression atomizers, nebulization inhalation consumables, suctioners, sleep respiratory therapy devices, infrared thermometers, and related accessories. At present, the company has more than 150 employees and a monthly production capacity of 150000 units.The enterprise adheres to the quality policy of "free breathing, national health" and continuously strives to innovate and develop healthy respiratory products, providing the best therapeutic effect for respiratory diseases for patients worldwide.The enterprise adheres to the principle of "technology builds brand, quality builds reputation" and ensures the provision of highly reliable medical device products to global customers through innovation, quality, reputation, and service.The company currently holds more than 30 patents and has passed SGS ISO13485 medical device quality system certification, EU CE product certification, and domestic GMP medical device quality system certification. In 2010, it was awarded as a qualified supplier by Disney, and in 2017, it passed the US FDA audit as an unobserved enterprise in one go.The company strictly implements domestic and international quality system laws and regulations, and its products are exported to more than 50 countries and regions including the United States, Russia, Germany, Italy, Brazil, the Middle East, Southeast Asia, etc., receiving recognition and praise from domestic and foreign users and merchants.The CE MDR certification consulting service for compression atomizers and mesh atomizers provided by Rui Enni has successfully obtained the MDR certificate issued by SGS through the unremitting efforts of Rui Enni's professional teachers and the mutual cooperation of the enterprise (the certificate change is also the 16th certificate issued by Rui Enni's consulting agency). This marks that the company's products comply with the strictest compliance certification in the European Union, and also signifies that the company's products are listed in compliance. Wishing the enterprise to strive for excellence and achieve long-term success in product sales in the current environment!
709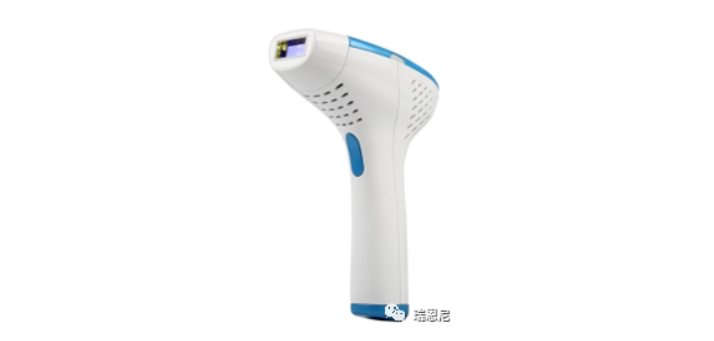
Good news! Rui Enni helps the same enterprise obtain approval for three 510 (k) zero defects The medical device 510 (k) registration project between Dongguan customers and Rui Enni has been successfully completed, and all three products have been approved with zero defects:The first IPL hair removal device: approved in April 2024, with no defects throughout the entire review process;Laser hair cap: submitted in early February 2025, efficiently approved in just 85 days;The second IPL hair removal device: Despite being extended to 98 days due to FDA auditor changes, it still maintains zero defect approval.All three registrations did not trigger formal issuance, demonstrating the team's professionalism and collaboration efficiency, and achieving the contract goals perfectly.Introduction to IPL Hair Removal Device The IPL hair removal device is used to permanently reduce adult hair regeneration. Full body usable (such as armpits, limbs, facial hair below the chin line)Working principle: The IPL hair removal device emits pulsed light of a specific wavelength, which is absorbed by melanin in dark hair and converted into heat energy, damaging hair follicles and papillae, and inhibiting hair regeneration.Classification: FDA Class II Medical Devices (Product Code: OHT) Introduction to Laser Hair CapUsage: Suitable for the treatment of adult androgenetic alopecia (AGA), stimulating hair follicles through low-energy laser therapy (LLLT) to promote hair regeneration.Working principle: The hair growth cap adopts LLLT technology, which can penetrate the scalp surface 3-5 millimeters deep and reach the hair follicle area through a low-energy laser of 5 milliwatts with a wavelength of 650 nanometers. Simultaneously inducing the secretion of endothelial growth factor, promoting the formation of new capillaries, providing richer nutritional supply for hair follicles, enhancing hair follicle cell activity, improving local blood circulation, thereby delaying hair loss and promoting hair regeneration.Category: FDA Class II Medical Devices (Product Code: OAP). Core difficulties:Regulatory compliance challenge: It is necessary to strictly meet the special control requirements of 21 CFR 878.4810 for phototherapy equipment, involving key tests such as optical safety and biocompatibility. The rate of addressing such issues in historical reviews is extremely high.Testing risk concentration: Optical safety (such as IEC 62471) and biocompatibility (ISO 10993) are key FDA review items, and similar products are often required to supplement data due to insufficient testing methods or data integrity.Breaking strategy:Accurately predict the review path:Collaborate with professional organizations such as Ryan Ni to intervene in advance, clarify product classification and compliance pathways through comparative analysis of predicate devices, and reduce review disagreements.Pre emptive resolution of supplementary risks:Based on the FDA's supplementary problem library for similar products, reverse optimize technical documents, supplement testing data in a targeted manner, and ensure "one-time submission pass".Establish an "immune document" system that simulates a closed loop of review logic (such as consistency between testing standards, data, conclusions, and label statements) to eliminate logical gaps.FDA 510 (K) Special List of Phototherapy ProductsOptical/Energy Safety Compliance:Compliant with IEC 62471 photobiological safety testing (blue light hazard, retinal thermal injury, etc.), IEC 60601-2-83 special requirements for household phototherapy equipment (energy limitation, fault protection), and IEC 60825-1 laser safety standards.Usability engineering verification:Simulate FDA human factors engineering scenarios, cover risk control verification of user misoperation (such as unexpected exposure, equipment tilt use), and ensure the effectiveness of instruction manual warnings.Equivalent Device Demonstration:Build a multidimensional comparative model (wavelength range, energy density, applicable parts), and demonstrate substantive equivalence (SE) with Predicte Device.Closed loop management of technical documents:Adopting the four-dimensional verification of "regulations standards testing documents" to ensure the closure of the data chain.Label/instruction manual compliance optimization: Revise warning language, contraindications, and symbol labeling in accordance with 21 CFR 801/809.10 standards to avoid issuing supplements due to vague wording.
787
Rui Enni helps a company in Jiangsu obtain the CE MDR certification for nutrition pipelines!Overcoming difficulties and working together to break through:CE MDR is the latest regulation issued by the European Union for medical devices, with clinical safety, technical compliance, and full lifecycle supervision as the core. The certification standards are strict and the process is complex. To assist enterprises in efficiently completing certification, our company has formed a professional team to provide full process consulting services, including product clinical evaluation, CE technical document optimization, and quality management system upgrade (adding MDR QMS).Accurate benchmarking of regulations: Assist enterprises in organizing MDR technical documents, improving risk management (ISO 14971), usability engineering (IEC 62366&IEC60601-1-6), and clinical evaluation evidence;Rapid response and rectification: Provide data supplementation and compliance strategy support for technical questions raised by the notified body (SGS1639);Milestone significanceThe passing of this certification not only demonstrates the technological leadership and international market competitiveness of the enterprise's products, but also sets a benchmark for China's medical device industry to break through the EU's technical barriers. In the future, this series of products will serve European medical institutions, benefit patients worldwide, and lay a solid foundation for enterprises to expand overseas markets.
695
Good news! Rui Enni Helps Nasal Inhaler Enterprises Obtain FDA 510 (k) Zero Issue Supplement Again, Certified in 43 Days!This FDA 510 (k) is the 35th zero subsidy case obtained by Ruini's assistance to enterprises!!1、 Introduction to electric nasal suction deviceThe electric nasal cannula is used to intermittently remove nasal secretions and mucus from children (2-12 years old). This device is designed for home environments.Working principle: The electric nasal cannula uses a motor pump to generate negative pressure in the suction system, allowing nasal secretions to flow into the device container. Classification: FDA Class II Medical Devices (Product Code: BTA) 2、 FDA 510 (k) registration experience of electric nasal cannulaDuring the application process for the 510 (k) electric nasal cannula, the Ruini team overcame multiple key challenges with their rich compliance experience and refined project management, ultimately achieving rapid approval with zero replacement. 3、 Registration experience sharing:Product manual: FDA regulations and guidelines are the cornerstone of 510 (k) registration, with detailed and strict requirements. When preparing registration materials, we conducted in-depth research on various relevant standards and guidelines. For example, product manuals must not only comply with electrical safety standards such as ANSI/AAMI ES 60601-1, but also follow FDA guidelines for patient labeling of medical devices. We need to describe the product characteristics and operating methods clearly, accurately, and concisely based on the unique design of the electric nasal cannula, ensuring clear and unambiguous logic. This requires us to cross check every item word for word to ensure compliance with every detail of the regulations.Packaging label: For the outer packaging color box of the product, it is necessary to strictly avoid exaggerating the product's functions and features, and ensure that the promotional content is true and reliable. The FDA attaches great importance to the authenticity of product advertising, and any false or misleading advertising may lead to registration failure. Therefore, when designing the outer packaging, we strictly describe the actual performance and characteristics of the product, without making any exaggerated statements.Performance testing: There is no ready-made industry standard for electric nose pumps abroad, which requires us to develop a testing report that fully complies with the product design and characteristics based on the product's characteristics and rich experience. In terms of performance testing, we cover multiple key indicators such as negative pressure accuracy, noise level, and battery life. Every testing method and requirement has been carefully considered and validated to ensure the accuracy and reliability of the test results.Data authenticity: In order to ensure the authenticity and integrity of the data, we cooperate with third-party laboratories with CNAS and GLP qualifications to conduct various tests that comply with standards.Compliance with standard checklist:Electrical Safety: ANSI/AAMI ES 60601-1Electromagnetic compatibility: IEC 60601-1-2Household environment: IEC 60601-1-11Biocompatibility: ISO 10993-1Software: IEC 62304Lithium battery safety: IEC 62133-2
542
Great news! Assisted Phototherapy Mask Successfully Approved for FDA 510 (k) In the field of medical device regulatory consulting, we continue to write brilliant chapters with our professional strength and unremitting efforts. Today, exciting news came: with the full assistance of our team, the customer's LED phototherapy mask has been successfully approved for FDA 510 (k)! This achievement is not only a powerful proof of the customer's product strength, but also a vivid reflection of our consulting service professionalism and efficiency.1. Introduction to LED Phototherapy MaskLED phototherapy mask is a household medical beauty device mainly used for treating wrinkles on the whole face and mild to moderate acne vulgaris. It cleverly utilizes specific wavelengths of light, including blue light (415nm), red light (630nm), and near-infrared light (830nm), to work in synergy. In acne treatment mode, blue light accurately sterilizes and red light assists in skin repair; In the wrinkle treatment mode, dual light synergistically stimulates collagen production, effectively reducing wrinkles and restoring skin radiance. The product design fully considers ergonomics, with an adjustable headband and a thermal compression function around the eyes (38 ℃ -41 ℃), providing users with a comfortable user experience. This product is quite popular in the markets of China, Europe, and America. It is classified as a Class II medical device in the United States and is marketed through 510 (k) registration. 2. FDA 510 (k) registration experience for LED phototherapy maskIn the early preparation stage of writing the application documents, we conducted comprehensive and in-depth research and analysis of the product with a rigorous attitude and professional spirit. To accurately grasp the core principles of the product, we extensively consulted a large number of authoritative professional literature materials. In the vast sea of information, professional knowledge and rich experience are used to carefully extract concise and clinically based descriptions of working principles, ensuring accurate and scientific expression that can withstand strict scrutiny.In the process of product parameters and testing, we closely integrate FDA guidelines and systematically classify products based on their structural characteristics. Conduct in-depth research on the various parameters that must be provided for this type of product, and carefully study scientific testing methods for different parameters. At the same time, strictly follow the FDA's guidelines on patient labeling and usability to carry out the design and preparation of product instructions. Not only that, but also carefully plan usability testing, covering key testing items such as tag readability, and ultimately issue professional and detailed testing reports.For every content and document that needs to be provided, we uphold an attitude of continuous improvement, from logical structure to written expression, we have repeatedly scrutinized and carefully polished them. Strive to achieve reasonable and coherent content, clear and coherent logic, and easy to understand expression, in order to accurately grasp every detail of the product and provide comprehensive documents, laying a solid foundation for the product to pass FDA review smoothly.3. Apply for key experience sharingBelow is a brief sharing of the problems and experiences encountered during the application process:In the current 510 (k) application process, there are relatively few overall supplementary issues, but there are also some key points. Among them, issues related to biocompatibility, the impact of light on the eyes, and heating modules are particularly prominent.In terms of biocompatibility, as the product is used to treat mild to moderate inflammatory acne, the FDA requires additional biocompatibility testing items related to damaged skin for this purpose. However, based on the FDA's past approval records, similar products do not have consistent practices in this regard. Some similar products have been tested for damaged skin, while others have not undergone this test. Even in the absence of testing on damaged skin using comparable devices with identical expected uses, the FDA still insists on imposing this requirement on our product. This phenomenon fully demonstrates that the FDA's requirements for biocompatibility are becoming increasingly strict, and also reflects the differences in the focus of different auditors. It is worth mentioning that our company, with rich experience and professional prediction, anticipated the possible occurrence of this issue before testing. Therefore, we conducted the test in advance according to the intradermal stimulation standard and informed the customer in advance that there may be a need to supplement the test items according to the damaged skin, making sufficient preparations for the audit.In terms of heating modules, the FDA is highly concerned about their safety, focusing not only on the heating temperature itself, but also on the potential impact of temperature on tissues. This requires us to strictly control the heating module during product design and testing to ensure that it operates within a safe range and avoid potential harm to users.The impact of light on the eyes is also a key focus of the review. The FDA is particularly concerned about whether the product structure can effectively prevent eye damage from light. In addition to optimizing the product structure design, equipping users with goggles that fit the product structure is also a feasible solution to comprehensively ensure the eye safety of users during use.4. Join hands in the future and create more brilliance togetherThe successful approval of the LED phototherapy mask for FDA 510 (k) is another significant achievement of our consulting services. In the future, we will continue to deepen our expertise in medical device regulatory consulting, continuously improve our professional capabilities, and provide high-quality and efficient services to more customers. We look forward to working together with more enterprises to help more innovative medical devices smoothly enter the international market and contribute to the global healthcare industry.Congratulations once again on the successful approval of the FDA 510 (k) for the LED phototherapy mask! We look forward to this product achieving outstanding results in the international market and bringing beauty and health to our users!
542Phone
0755-27391220
020-82513196

WeChat customer service

Mini Program
reanny@reanny.com