Good news! Rui Enni helps the same enterprise obtain approval for three 510 (k) zero defects
The medical device 510 (k) registration project between Dongguan customers and Rui Enni has been successfully completed, and all three products have been approved with zero defects:
The first IPL hair removal device: approved in April 2024, with no defects throughout the entire review process;
Laser hair cap: submitted in early February 2025, efficiently approved in just 85 days;
The second IPL hair removal device: Despite being extended to 98 days due to FDA auditor changes, it still maintains zero defect approval.
All three registrations did not trigger formal issuance, demonstrating the team's professionalism and collaboration efficiency, and achieving the contract goals perfectly.
Introduction to IPL Hair Removal Device
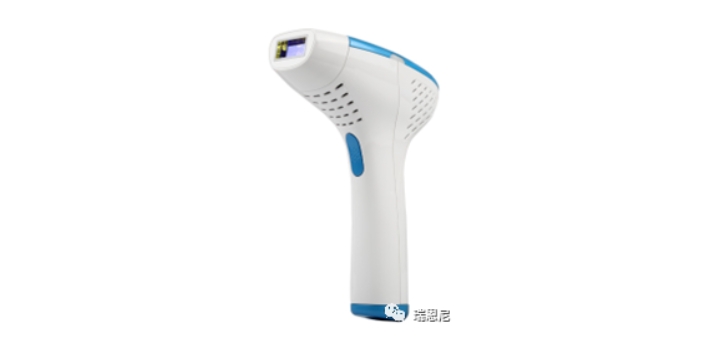
The IPL hair removal device is used to permanently reduce adult hair regeneration. Full body usable (such as armpits, limbs, facial hair below the chin line)
Working principle: The IPL hair removal device emits pulsed light of a specific wavelength, which is absorbed by melanin in dark hair and converted into heat energy, damaging hair follicles and papillae, and inhibiting hair regeneration.
Classification: FDA Class II Medical Devices (Product Code: OHT)
Introduction to Laser Hair Cap
Usage: Suitable for the treatment of adult androgenetic alopecia (AGA), stimulating hair follicles through low-energy laser therapy (LLLT) to promote hair regeneration.
Working principle: The hair growth cap adopts LLLT technology, which can penetrate the scalp surface 3-5 millimeters deep and reach the hair follicle area through a low-energy laser of 5 milliwatts with a wavelength of 650 nanometers. Simultaneously inducing the secretion of endothelial growth factor, promoting the formation of new capillaries, providing richer nutritional supply for hair follicles, enhancing hair follicle cell activity, improving local blood circulation, thereby delaying hair loss and promoting hair regeneration.
Category: FDA Class II Medical Devices (Product Code: OAP).

Core difficulties:
Regulatory compliance challenge: It is necessary to strictly meet the special control requirements of 21 CFR 878.4810 for phototherapy equipment, involving key tests such as optical safety and biocompatibility. The rate of addressing such issues in historical reviews is extremely high.
Testing risk concentration: Optical safety (such as IEC 62471) and biocompatibility (ISO 10993) are key FDA review items, and similar products are often required to supplement data due to insufficient testing methods or data integrity.
Breaking strategy:
Accurately predict the review path:
Collaborate with professional organizations such as Ryan Ni to intervene in advance, clarify product classification and compliance pathways through comparative analysis of predicate devices, and reduce review disagreements.
Pre emptive resolution of supplementary risks:
Based on the FDA's supplementary problem library for similar products, reverse optimize technical documents, supplement testing data in a targeted manner, and ensure "one-time submission pass".
Establish an "immune document" system that simulates a closed loop of review logic (such as consistency between testing standards, data, conclusions, and label statements) to eliminate logical gaps.
FDA 510 (K) Special List of Phototherapy Products
Optical/Energy Safety Compliance:
Compliant with IEC 62471 photobiological safety testing (blue light hazard, retinal thermal injury, etc.), IEC 60601-2-83 special requirements for household phototherapy equipment (energy limitation, fault protection), and IEC 60825-1 laser safety standards.
Usability engineering verification:
Simulate FDA human factors engineering scenarios, cover risk control verification of user misoperation (such as unexpected exposure, equipment tilt use), and ensure the effectiveness of instruction manual warnings.
Equivalent Device Demonstration:
Build a multidimensional comparative model (wavelength range, energy density, applicable parts), and demonstrate substantive equivalence (SE) with Predicte Device.
Closed loop management of technical documents:
Adopting the four-dimensional verification of "regulations standards testing documents" to ensure the closure of the data chain.
Label/instruction manual compliance optimization: Revise warning language, contraindications, and symbol labeling in accordance with 21 CFR 801/809.10 standards to avoid issuing supplements due to vague wording.




