Good news: Guangzhou Zhuyitong has successfully approved the launch of another household radiofrequency skin treatment device
On September 5, 2025, the third type of medical device "radiofrequency skin therapy device" developed and produced by Prometheus Miracle (Shenzhen) Medical Technology Co., Ltd. has been officially approved by the National Medical Products Administration, with registration certificate number: National Medical Device Approval No. 20253091825. This project is fully managed by our company Guangzhou Zhuyitong Pharmaceutical Consulting Co., Ltd. (hereinafter referred to as "Zhuyitong") throughout the entire clinical trial process, from project initiation to completion, all independently managed by Zhuyitong. This project is the first approved handheld three function (RF, LED, and EMS) RF skin therapy device. This approval marks the second certificate of the radiofrequency skin therapy device for clinical trial services provided by Guangzhou Zhuyitong.
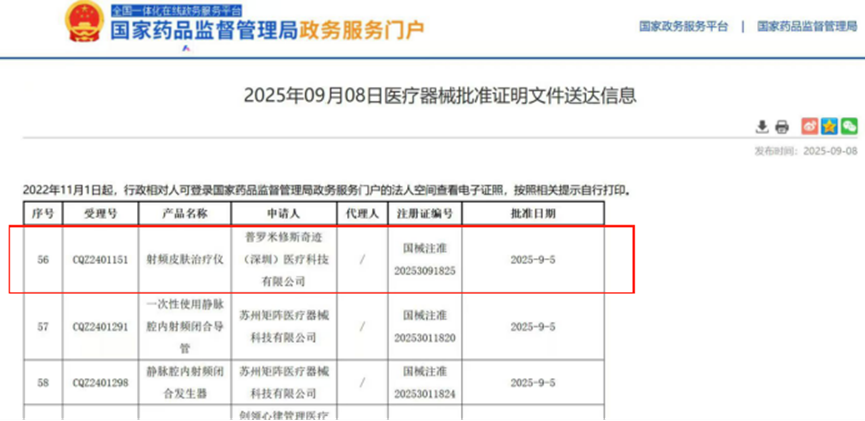
This is not only a recognition of the customer's product capabilities, but also an indirect recognition of our team's hard work and professional abilities! The following is the experience sharing of Zhuyitong on this clinical trial:
1、 Project Background
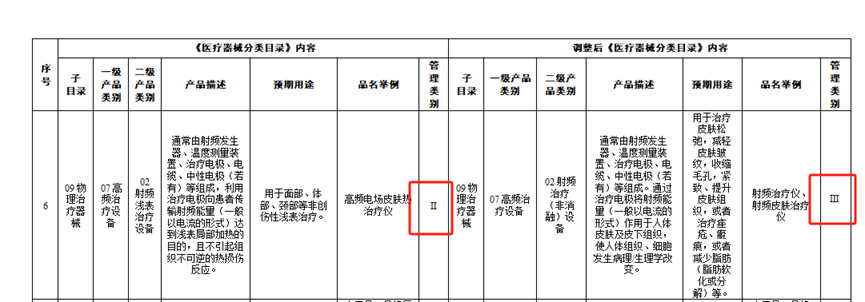
With the expansion of the medical beauty market, relevant departments in China have also standardized and regulated the medical beauty industry to ensure its healthy development. On March 30, 2022, the National Medical Products Administration issued a notice (No. 30 of 2022) on adjusting some contents of the "Classification Catalogue of Medical Devices", which includes adjustments to the contents of the "Classification Catalogue of Medical Devices" for 27 categories of medical devices. According to the relevant requirements of this announcement, radiofrequency beauty equipment is clearly managed as a Class III medical device. For the radio-frequency skin therapeutic instrument products in the 09-07-02 radio-frequency treatment (non ablation) equipment involved in the adjustment in the annex, from the date of the announcement, it is necessary to apply for registration in accordance with the Administrative Measures for the Registration and Filing of Medical Devices (Order No. 47 of the State Market Supervision and Administration). Starting from April 1, 2024, radiofrequency skin therapy devices cannot be produced, imported, or sold without obtaining a medical device registration certificate in accordance with the law. Starting from April 1, 2024, enterprises that have not obtained a medical device production and operation license (filing) are not allowed to engage in the production and sale of related products.
In April 2023, the Medical Device Technical Evaluation Center of the National Medical Products Administration issued the "Guiding Principles for Registration and Review of Radiofrequency Beauty Devices". The clinical evaluation requirements for radiofrequency beauty devices in the guiding principles should refer to the recommended path for clinical evaluation of products related to "Physical Therapy Devices" in subdirectories 09 of the "Classification Catalogue of Medical Devices". Recommend conducting relevant evaluations through clinical trial pathways.
At this critical juncture of regulatory upgrading in the industry, Zhuyitong has been entrusted by Prometheus Miracle (Shenzhen) Medical Technology Co., Ltd. with full responsibility for the clinical trial project of its radiofrequency skin therapy device, based on its professional reputation. This fully demonstrates the applicant's deep trust in Zhuyitong.
2、 Project milestones
The clinical trial of this project lasted approximately one year from the finalization of the protocol design to the release of the trial summary report. From the initial stage of the project's clinical trial plan design, the team collaborated with multiple medical beauty clinical experts and data statisticians to conduct multiple rounds of argumentation, repeatedly optimizing inclusion/exclusion criteria, evaluation indicators, and sample size calculation models to ensure that the plan not only meets the requirements of the "Guidelines for Registration and Review of Radiofrequency Beauty Equipment", but also has clinical operability; Screening and conducting research at the center, inspecting the medical beauty departments of multiple tertiary hospitals across the country, and ultimately determining two clinical centers that meet the requirements; In the ethical approval stage, actively cooperate with the hospital's ethical requirements to submit materials, complete the ethical meeting of the team leader unit within one month and obtain approval; And the subsequent center launch meeting organization, full cycle management of subjects (with a cumulative follow-up dropout rate of only 1.4%), third-party inspection cooperation (no major quality issues), data statistical analysis, until the writing of the summary report and submission of registration materials, each link embodies the hard work of the Zhuyitong team.
During the project promotion process, as the handheld radiofrequency skin therapy device was first placed under medical device supervision, there was neither consensus among clinical experts nor clinical precedents to refer to in China. During the process of scheme design and review, relevant parties actively communicated and cooperated, successfully completing all clinical trials and review Q&A, and ultimately obtaining approval.
3、 Project milestones
At the end of November 2024, the project will undergo on-site inspection by the National Medical Products Administration. Zhuyitong dispatched all trial participants to provide on-site assistance throughout the entire process, offering professional technical support and process coordination to ensure efficient and smooth verification work, further confirming the team's professional ability and responsible attitude.

Figure 1: Welcome Meeting for On site Verification by Relevant National Regulatory Authorities

Figure 2: Medical Assistance Team Members Assist in On site Verification
The passing of GCP inspection marks that the clinical trial process of Zhuyitong conforms to the principles of standardization and science, and can withstand the review of relevant parties and regulators. This is an example that proves the standardization of clinical management in Zhuyitong, and it is also a good start. It is also a reference example for more clinical trial projects in the future, and a sign of progress and continuous improvement in the future.
3、 Conclusion
The successful approval of a medical device relies on the collaborative efforts of the sponsor, clinical research unit, CRO, and SMO teams. Zhuyitong is fortunate to be a key participant, providing professional services to sponsors and clinical research units. In the future, it will continue to leverage the professional advantages of CRO clinical services and registration to support the development of the medical beauty industry!
In the journey of innovative medical devices from the laboratory to the market, we are well aware of the challenges that enterprises face: complex regulations, rigorous clinical validation, and lengthy approval cycles. As a deep vertical medical device CRO service organization, our company is willing to become your most reliable strategic partner, providing full cycle solutions from research and development planning to successful registration.
Forward planning and risk reduction: In the early stages of product development, we intervene to provide registration strategy consulting and clinical pathway planning to clarify your direction and avoid fatal defects in the technical review stage.
Efficient execution and quality assurance: We have a strong team of experts and execution network, providing one-stop management services for clinical trials, including protocol design, center screening, ethical approval, monitoring and inspection, data management and statistics, to ensure compliance in the trial process and solid data.
Successful application, perfect conclusion: We are proficient in registration regulations and can efficiently write and integrate key application materials such as clinical evaluation reports (CERs) for you. We also provide professional guidance for examination preparation, greatly improving the success rate of registration.
We not only deliver projects, but also deliver success and peace of mind. Based on the successful cases of previous projects, we promise to provide the highest professional standards and the most responsible attitude to escort your innovative products throughout the process, helping them to be launched as soon as possible, benefiting patients, and winning commercial success.
At present, the clinical trial projects being carried out by Zhuyitong include IPL hair removal device, hearing aids, HIFU products, blood oxygen, etc. Zhuyitong has a professional and experienced clinical trial team, including PM, clinical medicine, statistics, CRA, and SMO.
Contact us immediately for exclusive listing solutions!




