
Mechanical root canal file is a dental rotary instrument that needs to be used in conjunction with a dental mobile phone to provide power. The machine used root canal file consists of a handle and a head working end. The body of the file is made of nickel titanium alloy, and the working end has grooves or spiral cutting edges. It is used for root canal preparation, auxiliary filling (i.e. cutting, leveling, cleaning, and shaping of dental bones, root canals, etc.) in dental treatment. The machine root canal file consists of an operating part and a rod, with a working part with a cutting edge on the operating part; The operating part is made of nickel titanium alloy material.Target users: The product must be used by dentists who have received formal training in root canal treatment. The expected usage location of this product is medical institutions, dental clinics, and similar occasions.Our company was commissioned by the client to handle the first registration of NMPA Class II medical devices for their company's machine root canal files at the end of December 2021. After 7 months, Rui Enni lived up to the customer's expectations and received the registration certificate approval on July 1, 2022. (Approval date: June 29, 2022, registration certificate number: Yue Xie Zhu Zhun 20222170852)After this registration, I have a few experiences to share with you:1) [Sensitive words] Step, first classify the root canal files according to their shape. The types of mechanical root canal files are complex, and according to their shape and taper, they can be classified into standard instruments, tapered instruments, molded instruments, non tapered instruments, and non single tapered instruments. The standard requirements corresponding to different classifications are also different.2) The second step is to carefully identify the type of root canal file within the applicable standards. Even for the same standard, each standard chapter should be carefully differentiated based on the different types of root canal files.3) Overall, from a professional perspective in the field of mechanical root canal filing, there are many shortcomings in the YY0803 sequence standard applicable to root canal filing. In terms of some content, most of the products that have already been launched do not actually meet some of the requirements of the YY0803 sequence standard. From the perspective of the reviewing teacher, mandatory requirements must be met. So, how to solve these contradictory problems is currently the challenge we are facing.4) There are many models and specifications of root canal files registered this time, with 56 registered models, and dozens of specifications are subdivided for different models. The product testing and issuance of test reports are also very patient tests. When testing at the provincial institute, the institute considers the model specifications to be large and is only willing to provide test reports for three main inspection models. All other models with differences in specifications need to be provided by the manufacturer themselves. Fortunately, due to policy changes, the drug regulatory authority can recognize the self testing reports of enterprises. This greatly saves product testing time.In summary, the NMPA registration of root canal files needs to focus on the compliance of standard terms and the consistency of similar compared products.
610
Rongfeng breast pump has obtained FDA 510K certification approvalK212884Electric breast pumps are classified as Class II medical devices in the United States Medical devices and products need to comply with the US FDA's regulations on medicine Requirements for medical equipment.The purpose of an electric breast pump is to allow lactating women to secrete and collect milk from their breasts. It is designed for individual users.The electric breast pump forms a sealed area around the nipple by sucking on accessories, and the air inside the sealed area is pumped away by the air pump, creating a vacuum and applying suction to the breast,Then release suction to stimulate lactation and extract milk. Milk is collected in the bottle.Due to the busy workload, more and more breastfeeding women choose to participate in work during their lactation period, and the use of electric breast pumps is safe Fully hygienic and convenient,Rongfeng has chosen to entrust us to handle the registration of their company's FDA 510K certification in the United States. Rui Enni fulfilled the client's commission and completed the approval of FDA 510K certification at the beginning of 2022.After this registration, there are several experiences that need to be shared, including:1) The breast pump is suitable for lactating women, single patient use, and needs to be cleaned and disinfected before use Poison;2) The testing of vacuum negative pressure needs to be ensured during operation;3) The instructions for using the breast pump need to comply with the requirements of the US Centers for Disease Control and Prevention.
696
Finger clip oximeters are a type of device in the United States Fang Medical Treat The main purpose of this device is to measure blood oxygen saturation (SpO2) and pulse through non-invasive methods at the fingertips.Due to the fine red color in the blood Cells, including oxygenated hemoglobin (HbO2) and reduced hemoglobin (Hb), are sensitive to red light (660nm) and red light Outside Line. (910nm) has different absorption abilities. Reduced hemoglobin (Hb) absorbs more red light Outside Line. Less. On the other hand, oxygenated hemoglobin (HbO2) absorbs less red light Outside Line. More. By setting the red LED and red at the same position on the nail oximeter Outside Line. LED lights, when light penetrates from one side of the finger to the other and is received by a photodiode, can generate a corresponding proportion of voltage. After being processed by the microcontroller, the output result is displayed on the LCD screen.During the epidemic, it was one of the five major components for epidemic prevention, along with masks, thermometers, oxygen concentrators, and ventilators. So during the epidemic, a large number of companies competed to register for certification of blood oxygen meters for export to Europe and America. After comparing with multiple peers, Lingxin Company was finally recognized by our experts Moved by business and sincerity, they chose to entrust us to handle their company's EU CE and US FDA 510K certification registration. Rui Enni fulfilled the client's commission and completed its CE certification before the first half of 2021, and obtained the FDA 510K certification approval by the end of 2021.After this registration, there are several experiences that can be shared, including:1) Confirm if the product claims to be suitable for children? If applicable to children, temporary Bed accuracy testing needs to consider appropriate samples; At the same time, consideration should be given to the duration of continuous use and prevention measures Stop the blood circulation caused by long-term pressure on the fingers Environmental issues;2) Confirm if the product is intended for low perfusion situations? If so, specific requirements of standards or guidelines should be considered for this;3) If the product has wireless functionality, the app settings and overall product planning need to meet the requirements of relevant countries Home network security Complete basic requirements;4) Suggestion for Lin Bed accuracy testing and consulting machine Structure Okay. Choose the same company to avoid arguing with each other; Lin Bed testing must meet the program requirements of GCP;5) When the product exceeds the effective range, an alarm prompt should be displayed, and values outside the effective range should not be displayed to avoid misunderstandings for users.6) Many companies' oximeters are purchased from solution providers, so it is important to confirm the maturity of the solution provider's products in the market. Many solution providers' designs have not undergone actual implementation Bed verification can lead to inaccurate measurements at the low oxygen level. At the same time, during the certification process, it is necessary for the solution provider to receive a quick response (such as when software or design modifications are needed) in order to ensure that the certification is passed quickly.
513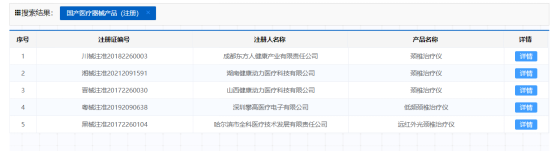
The cervical spine treatment device has emerged in the past three years and is more suitable for white-collar workers. In recent years, brands with good operations have emerged continuously, such as SKG and Pangao. The promotion of cervical spine therapy equipment focuses on its stylish appearance, but performance is the key to obtaining a good after-sales reputation. The positioning of medical devices themselves is to have certain medical characteristics and uses, not just electronic products for gaming.Based on its strong pharmaceutical background, Miaobang Company is positioning itself and entering the cervical spine treatment device project, with the expectation of obtaining NMPA registration in China. The customer contacted our company through Baidu search, and after a face-to-face discussion, the customer decided to choose us. For me, I used to work in a company for 5 years producing physical rehabilitation medical devices, and our expertise lies in therapeutic equipment products. I still remember that in 2018, we achieved zero defect approval for two consecutive stimulators.During the application process, it took some time to rectify the inspection, so the important lesson here is that when companies enter the medical device industry, they must hire professional R&D and regulatory personnel with medical industry experience. The development of medical devices should be combined with standard design in design planning, rather than self-awareness or blind reference to marketed devices. This is the current situation in the industry. Many companies do not consider the need for verification and certification during development, and only realize that the design cannot meet the requirements during verification and certification. They repeatedly make design changes, which are time-consuming, expensive, and laborious.With the good cooperation of both parties in the later stage, we successfully passed the registration review and physical examination, and passed the NMPA registration. During the registration review, supplements were made due to issues with the product name. According to the new understanding of the National Medical Products Administration, medical devices should use a common name, so even if the cervical treatment device can only be used for neck treatment, it is still required to change to the common name of low-frequency treatment device. In the early market, similar products that had already been approved included: So the experience of this NMPA certification process is:1) Policies vary in different provinces; The scale of the same province is constantly changing and gradually becoming standardized;2) Priority should be given to selecting products from the same province within the past three years based on similar medical devices;3) Keep abreast of new regulations and policy trends in a timely manner, and maintain cognitive synchronization.
515
At the beginning of 2022, the results of the project were received - Taikang Medical Doppler Fetal Aspirator obtained FDA 510K certification approval with K number 211940.The client is a start-up medical device enterprise located in Pingshan. This project is an FDA 510K certification application submitted to the United States. The client attaches great importance to it, and after a round of comparison in the consulting circle in Shenzhen, they finally chose Rui Enni Consulting based on the recommendation of the testing laboratory and the promotion of high cost-effectiveness. At the beginning of the project, our consultant planned suitable inspection items for the client and assisted them in reviewing and providing relevant documents before the report was issued. Throughout the entire audit process, a round of supplements was made. In addition to the routine descriptive issues in the explanations, the auditors also made some audit findings and recommendations on the security report, performance report, and EMC report. At the same time, auditors pay special attention to the safety of batteries. In addition to testing reports, they also require a quality control plan for the battery production process. These issues are quite common for us, and we promptly analyzed and completed the rectification after receiving the audit findings. The FDA510K certification for the project was originally expected to be approved by the end of December 2021, but was postponed to early January 2022 due to the Double Dan Festival in the United States. Okay, let's start the new year with a beautiful outcome.
628
Congratulations to KWL for successfully obtaining the 510 (K) approval for wrist blood pressure monitors. The wrist blood pressure monitor went through more than two months from application to approval, and was approved after a round of rectification and improvement. Some of the supplementary content mainly involves partial revisions of third-party testing reports, software documentation, and manuals. After receiving the rectification requirements, we Notify the customer immediately to find a testing machine We will revise the report and be responsible for rectifying the remaining issues. Once the client receives the revised third-party testing report, we will immediately address it Submit all rectification materials to FDA as required. We received an email notification from the FDA regarding the approval of 510K within one month of submission.It only takes about two months from submission to approval, which is inseparable from our registered teachers' familiarity and control of the product, regulations, audit requirements, and key points.Ryan always uses expertise Our service attitude of professionalism, dedication, and high response speed helps customers quickly obtain certificates legally and compliantly, accelerate listing, and achieve economic benefits Great expansion!
523Phone
0755-27391220
020-82513196

WeChat customer service

Mini Program
reanny@reanny.com