Background Introduction
With the continuous improvement of the living standards of the domestic people, their pursuit of beauty is unprecedentedly high. Based on market demand, a large number of household beauty devices have emerged, such as IPL hair removal devices, phototherapy products, radiofrequency beauty devices, Therma, etc. Based on the changing demand in this market, in order to reasonably control the risks of beauty devices, the Food and Drug Administration is gradually referring to the regulatory model of the US FDA and incorporating beauty devices as medical beauty devices into the scope of medical devices for management in batches.
In March 2022, the bureau officially issued a notice requiring the inclusion of radiofrequency beauty devices in medical device supervision and their entry into the market under medical device licensing from April 1, 2024.
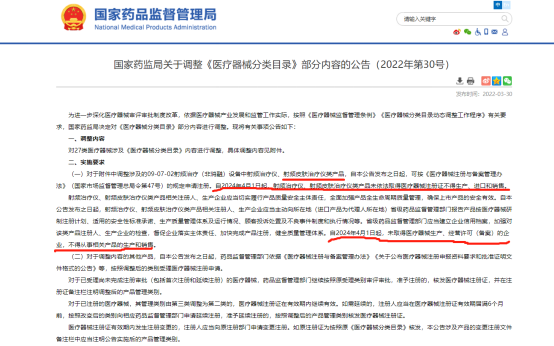
In April 2022, the bureau issued a notice soliciting opinions on the "Guiding Principles for Classification and Definition of Radiofrequency Beauty Products" (draft for comments). RF beauty devices are classified as follows:

In July 2022, the bureau officially proposed classification and definition opinions for radiofrequency beauty products:




Medical beauty is a new opportunity and trend in the development of medical devices. Based on this, Guangdong Province has issued relevant opinions on supporting the development of the medical beauty industry - the "Letter from the Office of the Guangdong Provincial Health Commission on Soliciting Opinions on Medical Beauty Surgery Projects and Their Graded Management Catalogue in Our Province" (hereinafter referred to as the "Draft for Soliciting Opinions").
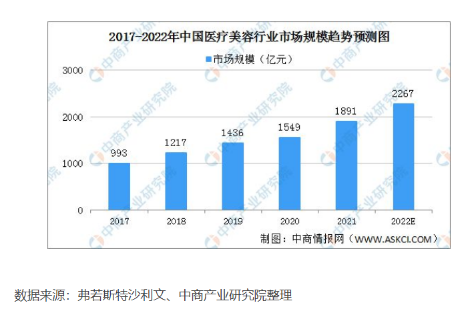
With China's economic growth and consumption upgrading, the medical beauty industry in China has developed rapidly, and its market size has jumped to second place. The industry has great development space and fierce market competition. The market size of China's medical beauty market has increased from 99.3 billion yuan in 2017 to 1891 billion yuan in 2021, with a compound annual growth rate of 17.5%. The growth rate of China's medical beauty market is much higher than the total market, and it is expected that the market size of China's medical beauty market will reach 226.7 billion yuan in 2022.
Section 2: Strong Alliance
Rui Enni Consulting has been working in the medical device consulting industry for 9 years and has rich experience in domestic and international registration of medical devices. At present, there are 20 full-time consultants with complete project service capabilities (including design review for domestic and international markets, factory specifications, registration agency, system counseling, clinical trials, production licensing, education and training, and long-term consulting services).
The customer registered for the NMPA radiofrequency skin therapy device was established in 2003 and is one of the earliest high-tech enterprises in China focused on the medical beauty industry. The enterprise has numerous high-quality talents with strong capabilities in design and development, quality management, and manufacturing, providing ODM and OEM services for top domestic and foreign brands.
In the context of medical beauty enterprises, the client company has decided to register Class III radiofrequency skin therapy devices based on their own development needs. After multiple rounds of negotiations and discussions with various consulting firms, the client ultimately chose Rui Enni Consulting. This registration project is an important "iconic Class III medical device registration project" for Ruienni in the field of medical beauty devices. Ruienni is determined to make this RF project a golden model for enterprise services. It marks an important milestone in the development of Ruini in 2022; It also marks Ryan's active progress towards higher, faster, and more professional directions. This project has significant strategic importance for Rainey.

At the beginning of the project kickoff meeting, the client's project representative delivered a warm speech. During the process, the customer representative explained the strategic significance of this registration project from the perspective of the enterprise itself, as well as the challenges and opportunities that the enterprise will soon face.
Mr. Wang, the founder of Ruini, was also invited to deliver a speech. The project was summarized from various aspects such as the difficulty of product registration, registration plan, and project service team, and received warm applause from the client.
At the end of the meeting, the founder of Ruini (left in the picture below) and the general manager of the client company (right in the picture below) signed the "NMPA Registration Agency Consulting Contract for RF Skin Treatment Equipment", and the project was officially launched.

Section 3: Outlook
After the project is signed, the next step will be for all members of the Ruini project team to participate in the service process. I believe that with mutual trust, sincere cooperation, and efforts from both parties, we can obtain the Class III product registration certificate and production license within the target plan.
When we receive good news in the future, we will publish another article to report it to everyone!!!




