The cervical spine treatment device has emerged in the past three years and is more suitable for white-collar workers. In recent years, brands with good operations have emerged continuously, such as SKG and Pangao. The promotion of cervical spine therapy equipment focuses on its stylish appearance, but performance is the key to obtaining a good after-sales reputation. The positioning of medical devices themselves is to have certain medical characteristics and uses, not just electronic products for gaming.
Based on its strong pharmaceutical background, Miaobang Company is positioning itself and entering the cervical spine treatment device project, with the expectation of obtaining NMPA registration in China. The customer contacted our company through Baidu search, and after a face-to-face discussion, the customer decided to choose us. For me, I used to work in a company for 5 years producing physical rehabilitation medical devices, and our expertise lies in therapeutic equipment products. I still remember that in 2018, we achieved zero defect approval for two consecutive stimulators.
During the application process, it took some time to rectify the inspection, so the important lesson here is that when companies enter the medical device industry, they must hire professional R&D and regulatory personnel with medical industry experience. The development of medical devices should be combined with standard design in design planning, rather than self-awareness or blind reference to marketed devices. This is the current situation in the industry. Many companies do not consider the need for verification and certification during development, and only realize that the design cannot meet the requirements during verification and certification. They repeatedly make design changes, which are time-consuming, expensive, and laborious.
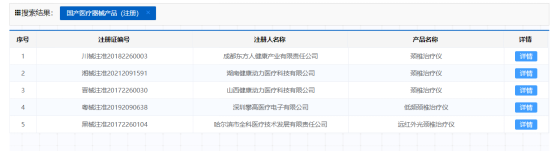
With the good cooperation of both parties in the later stage, we successfully passed the registration review and physical examination, and passed the NMPA registration. During the registration review, supplements were made due to issues with the product name. According to the new understanding of the National Medical Products Administration, medical devices should use a common name, so even if the cervical treatment device can only be used for neck treatment, it is still required to change to the common name of low-frequency treatment device. In the early market, similar products that had already been approved included:
So the experience of this NMPA certification process is:
1) Policies vary in different provinces; The scale of the same province is constantly changing and gradually becoming standardized;
2) Priority should be given to selecting products from the same province within the past three years based on similar medical devices;
3) Keep abreast of new regulations and policy trends in a timely manner, and maintain cognitive synchronization.




