
Rui Enni has helped a company in Beijing obtain CE MDR certification for nutrition pumps, injection pumps, and infusion pumps!Overcoming difficulties and working together to break through:CE MDR is the latest regulation issued by the European Union for medical devices, with clinical safety, technical compliance, and full lifecycle supervision as the core. The certification standards are strict and the process is complex. To assist enterprises in efficiently completing certification, our company has formed a professional team to provide full process consulting services, including product clinical evaluation, CE technical document optimization, and quality management system upgrade (adding MDR QMS).Accurate benchmarking of regulations: Assist enterprises in organizing MDR technical documents, improving risk management (ISO 14971), usability engineering (IEC 62366&IEC60601-1-6), and clinical evaluation evidence;Rapid response and rectification: Provide data supplementation and compliance strategy support for technical questions raised by the notified body (SGS1639);Milestone significanceThe passing of this certification not only demonstrates the technological leadership and international market competitiveness of the enterprise's products, but also sets a benchmark for China's medical device industry to break through the EU's technical barriers. In the future, this series of products will serve European medical institutions, benefit patients worldwide, and lay a solid foundation for enterprises to expand overseas markets.
592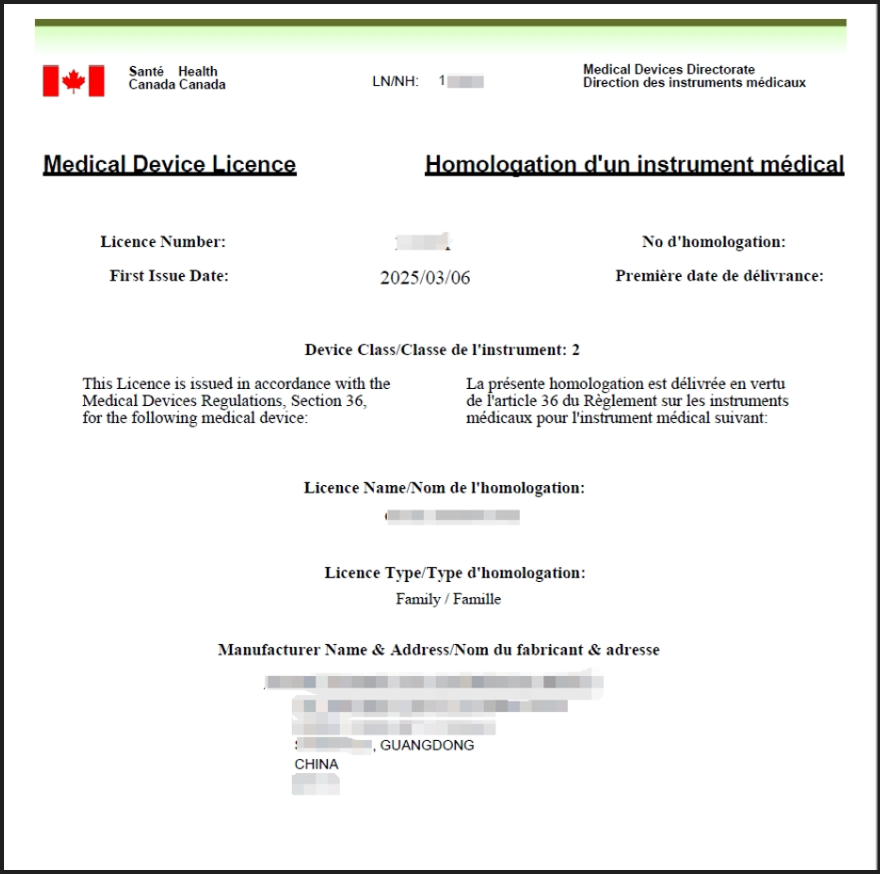
XX Technology, founded in 2006, is a world leading global enterprise in the research and manufacturing of oral health products. It is a national high-tech enterprise and a permanent member of the China Household Appliances Association. We specialize in researching electronic oral care products such as electric toothbrushes, dental irrigators, teeth whitening devices, and ultraviolet disinfectors. Since its establishment, it has continuously invested a large amount of research and development funds, with over 100 R&D engineers and more than 500 patents. It has production bases in Shenzhen, Guangdong, Nanchang, Jiangxi, and Haiphong, Vietnam, with a total area of over 120000 square meters and more than 2600 employees. XX Technology has passed the certification of ISO 9001:2015 Quality Management System, ISO 13485:2016 Medical Device Quality Management System, MDSAP, BSCI, WCA and other relevant industry system standards at home and abroad, and has obtained the GS1 certificate and Dun&Bradstreet Enterprise Code. The product has passed FCC, CE, CB, ETL and other certifications, and is registered with the FDA and the US Food and Drug Administration. With cutting-edge technology, a sound manufacturing system, and cost advantages in overseas factories, XX Technology provides customers with technologically advanced, quality compliant, and cost-effective product services. Since its establishment, it has gained the trust of globally renowned brands and dental clinics, and its products are sold to more than 30 countries and regions.Rui Enni assisted the company in applying for MDL registration in Canada for dental irrigators. The application materials were submitted on February 24, 2025, and the certificate was obtained on March 6, 2025. The certificate was obtained in only 10 days with zero issues and no supplements throughout the process!
715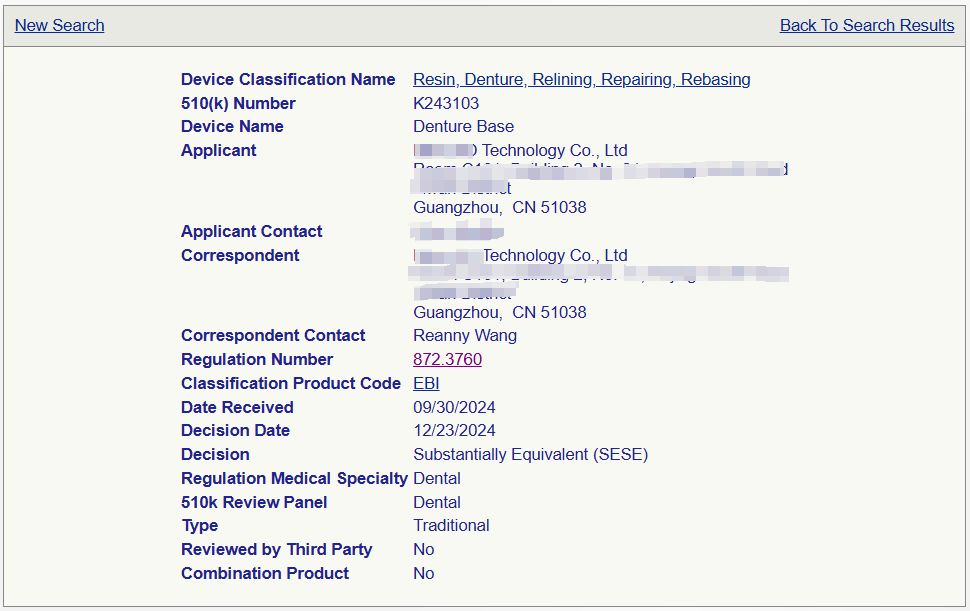
Congratulations to Rui Enni for assisting a customer in Guangzhou in obtaining 510 (k) zero replacement for their 3D additive printed denture base resin, which took 2 and a half months to obtain certification;This application starts with assisting the enterprise in planning various product testing and obtaining Small Business (SBD) certification; And expedite the submission of eStar by the end of fiscal year 2024, successfully obtaining 510 (k) approval with zero defects after 2 and a half months. Saved the enterprise from the increased 510 (k) application fee due to the 2025 fiscal year
928
Case Company Introduction: XX Technology (L~~~~) is a digital 3D intelligent manufacturer specializing in the research and development of 3D printing equipment, software, and materials, committed to changing the way products are developed and produced. The team has developed high-performance material systems suitable for different industries, relying on independently developed XX series printers and supporting software to provide solutions for innovation and upgrading in industries such as footwear, dentistry, healthcare, consumer, and automotive. They aim to create a new digital manufacturing model and ecological loop that combines customization and mass production.Rui Enni provided MDSAP certification coaching services for the company established in Silicon Valley, USA (USA+Canada+Australia+Japan). We provided remote tutoring for 3 months and successfully obtained the MDSAP certification.
865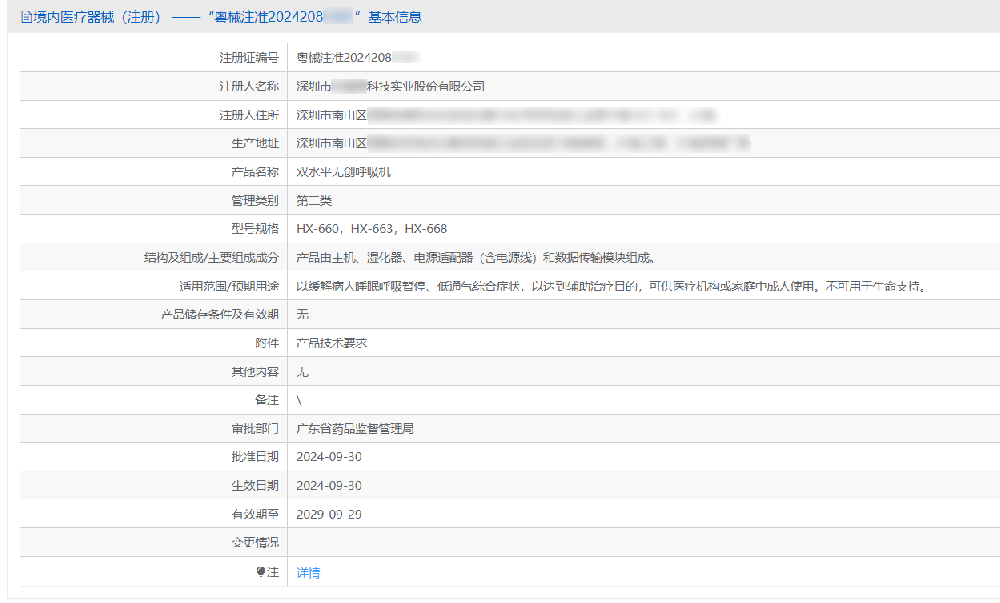
Shenzhen Science and Technology Industrial Co., Ltd. was established in 1991. It is a national high-tech enterprise that integrates professional independent design, research and development, production, and sales of various measuring instruments and meters. It is the president unit of the Shenzhen Instrumentation and Automation Industry Association, the vice president unit of the China Indoor Environment Purification Industry Alliance, the vice president unit of the Guangdong Indoor Environmental Health Industry Association, a participating unit in the "Classification of Indoor Air Quality for Infants and Young Children" standard and the "Classification of Particle Purification Performance of Filter Air Purifiers" standard, and the drafting unit of the "Infrared Human Surface Temperature Rapid Screening Instrument" national standard.The enterprise always adheres to quality first, continuously improves its management system, and gradually establishes a sound product quality management system. Since 2004, it has passed ISO9001 quality certification and ISO13485 medical quality system certification, meeting customers' requirements for product quality. The company has won honors such as "National High tech Enterprise", "Top 100 Taxpaying Enterprises", "Shenzhen Famous Brand" and "Leading Enterprise" in its development process.Rui Enni's assistance to the company in obtaining the "Dual level Non invasive Ventilator" lasted for 10 months. We also wish the company smooth sailing and Yang Fan moving forward!
1147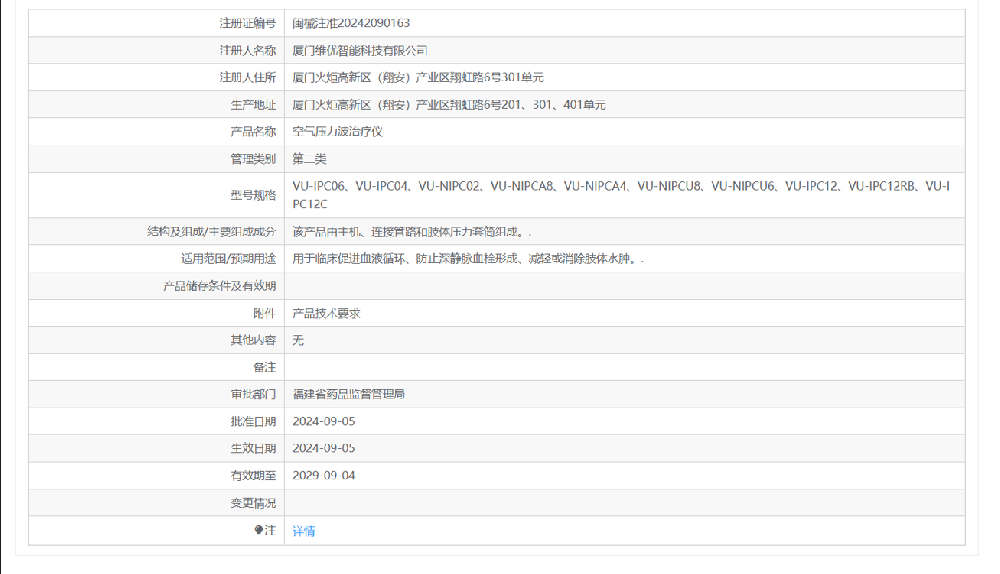
Xiamen Intelligent Technology Co., Ltd. utilizes its unique advantages to focus on providing customers with comprehensive services in rehabilitation therapy, healthcare equipment, and smart consumer electronics products. Our team has extensive experience in the fields of rehabilitation and intelligent devices, with strong research and development capabilities. We innovate based on the standards of "high standards, high quality, high efficiency, high safety, and high performance", and strive to explore the development direction of leisure life. We are determined to create a cross industry ecosystem in the field of leisure life and provide a comprehensive solution for "maintaining life baselines and optimizing life character" for the global leisure life community.The company's main products include air pressure therapy equipment series, medical transfer bed series, nursing mattresses, and DVT (deep vein thrombosis therapy equipment) series. The product quality is excellent, the price is reasonable, the design is novel, the materials are environmentally friendly, and the service is sincere, winning the trust of domestic and foreign customers.Our product has become one of the professional brands with influence and competitiveness in the same industry.Rui Enni helped the company's pneumatic therapy device obtain the domestic Class II medical device registration certificate, which took a total of 9 months.
1055Phone
0755-27391220
020-82513196

WeChat customer service

Mini Program
reanny@reanny.com