Good news!
Rui Enni Helps Nasal Inhaler Enterprises Obtain FDA 510 (k) Zero Issue Supplement Again, Certified in 43 Days!
This FDA 510 (k) is the 35th zero subsidy case obtained by Ruini's assistance to enterprises!!
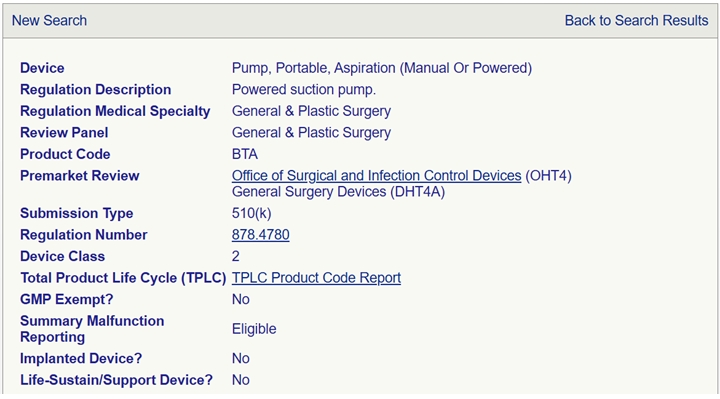
1、 Introduction to electric nasal suction device
The electric nasal cannula is used to intermittently remove nasal secretions and mucus from children (2-12 years old). This device is designed for home environments.
Working principle: The electric nasal cannula uses a motor pump to generate negative pressure in the suction system, allowing nasal secretions to flow into the device container.

Classification: FDA Class II Medical Devices (Product Code: BTA)
2、 FDA 510 (k) registration experience of electric nasal cannula
During the application process for the 510 (k) electric nasal cannula, the Ruini team overcame multiple key challenges with their rich compliance experience and refined project management, ultimately achieving rapid approval with zero replacement.

3、 Registration experience sharing:
Product manual: FDA regulations and guidelines are the cornerstone of 510 (k) registration, with detailed and strict requirements. When preparing registration materials, we conducted in-depth research on various relevant standards and guidelines. For example, product manuals must not only comply with electrical safety standards such as ANSI/AAMI ES 60601-1, but also follow FDA guidelines for patient labeling of medical devices. We need to describe the product characteristics and operating methods clearly, accurately, and concisely based on the unique design of the electric nasal cannula, ensuring clear and unambiguous logic. This requires us to cross check every item word for word to ensure compliance with every detail of the regulations.
Packaging label: For the outer packaging color box of the product, it is necessary to strictly avoid exaggerating the product's functions and features, and ensure that the promotional content is true and reliable. The FDA attaches great importance to the authenticity of product advertising, and any false or misleading advertising may lead to registration failure. Therefore, when designing the outer packaging, we strictly describe the actual performance and characteristics of the product, without making any exaggerated statements.
Performance testing: There is no ready-made industry standard for electric nose pumps abroad, which requires us to develop a testing report that fully complies with the product design and characteristics based on the product's characteristics and rich experience. In terms of performance testing, we cover multiple key indicators such as negative pressure accuracy, noise level, and battery life. Every testing method and requirement has been carefully considered and validated to ensure the accuracy and reliability of the test results.
Data authenticity: In order to ensure the authenticity and integrity of the data, we cooperate with third-party laboratories with CNAS and GLP qualifications to conduct various tests that comply with standards.
Compliance with standard checklist:
Electrical Safety: ANSI/AAMI ES 60601-1
Electromagnetic compatibility: IEC 60601-1-2
Household environment: IEC 60601-1-11
Biocompatibility: ISO 10993-1
Software: IEC 62304
Lithium battery safety: IEC 62133-2