Intense Pulsed Light (IPL)
Intense pulsed light (IPL) is a non laser light source composed of a single or multiple pulse sequence of incoherent light, characterized by a wide spectral range and high energy density. Short wavelength light can be filtered out using filters or coatings. For example, the 560 treatment head filter retains the spectrum within the wavelength range of 560nm to 1200nm, except for the band shorter than 560nm. The light source of the strong pulse light device is a xenon lamp, and its basic working principle is that a trigger applies high voltage to xenon gas to trigger xenon ionization. After being charged for a relatively long time through an energy storage capacitor, it discharges in a very short time, causing avalanche ionization of xenon gas inside the lamp tube. Xenon gas converts and releases the charged energy in the form of high-intensity light radiation, and this discharge process is a light pulse.
Using strong pulsed light with a certain penetration depth and good absorption ability in melanin, the hair follicles and hair shafts irradiated by light absorb a large amount of light energy, which is converted into heat energy and transmitted to the end of the hair follicles through the protein inside the hair, causing the entire hair to be heated. The protein and cells undergo coagulative necrosis, ultimately leading to the loss of the entire hair due to the necrosis of growing cells.
In the past, common hair removal methods in family environments included hair removal cream, wax paper, and hair scraper, which belonged to chemical or physical hair removal.

Hair removal wax paper uses brute force to remove hair, which not only causes strong pain, but also easily leads to folliculitis; The hair removal knife only removes the surface hair, so frequent scraping is needed to maintain it, and there is a risk of scratching; Hair removal cream belongs to chemical hair removal, which uses chemical substances to make hair fragile and prone to breakage, achieving the effect of hair removal. It is easy to cause skin allergies, has a short maintenance time, and has high long-term and large-scale use costs.
At present, the common hair removal equipment in hospitals or beauty institutions is generally a large multifunctional strong pulse light therapy device with a foot pedal.
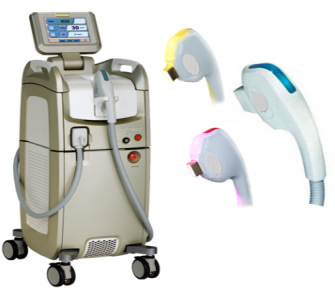
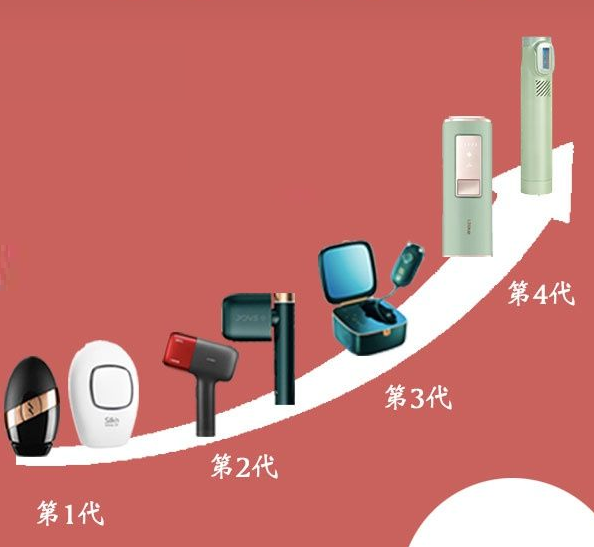
With the development of technology, IPL strong pulse light technology can be used in home environments and medical beauty institutions, and the equipment has become increasingly compact and portable through upgrades and iterations.
Recently, the strong pulse light therapy device assisted by Shenzhen Ruienni submitted an application according to the registrant system and was awarded one of the first batch of medical device registration certificates approved by the Guangdong Provincial Drug Administration for clinical evaluation (registration certificate number: Yuexizhun 20232090285).
During the registration process, the evaluation center focused on some key points with strong professional skills, such as the cut-off wavelength of the filter, the number of pulses, and the method of energy adjustment.
During the rectification process, a technical evaluation expert review meeting was held to address the differences in pulse energy and energy density between the proposed registered product and the comparison devices of the same variety. We have made sufficient preparations in advance before this meeting. During the meeting, we provided a comprehensive and detailed explanation and examples of the product's technical principles, structural materials, functions, safety and effectiveness related to clinical evaluation. The results of the meeting were unanimously recognized by the expert group.
Ruini lives up to the customer's trust! Within just over a month of submitting supplementary materials after the expert review meeting, we successfully obtained the registration certificate for the "Intense Pulse Light Therapy Device" issued by the Guangdong Provincial Drug Administration for clinical evaluation approval.
Here, we would like to share some points that need to be noted during the clinical evaluation and review process of selecting the same variety for NMPA registration:
When selecting the same variety for comparative analysis and clinical evaluation, it is necessary to focus on the technical key points of implementing various functions of the product, such as the wavelength range of terminal output pulse light, pulse energy, and energy density.
1. Wavelength range: In the wavelength range of most registered and marketed devices, the long wavelength can reach 1200nm, while some products have a long wavelength of only 950nm or even shorter, or longer than 1200nm. When submitting clinical evaluation terminals for comparative analysis of the same product, it is necessary to explain the safety and effectiveness of the product from both theoretical and clinical perspectives.
2. Pulse energy: Excessive pulse energy can cause varying degrees of adverse events. In comparative analysis, if the pulse energy of the device to be declared is higher than that of the equipment compared with the same variety, it is necessary to explain in detail the method of controlling the use of energy in the product, as well as the numerical changes in pulse energy and energy density to ensure the safe use of the equipment.




