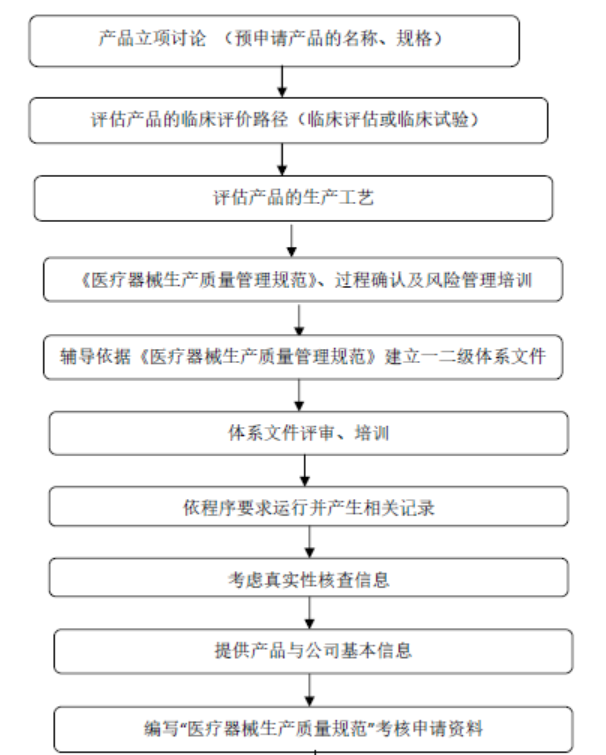
Coaching on domestic medical device GMP system
To strengthen the supervision and management of medical device production and standardize the quality management of medical device production, the China Food and Drug Administration (CFDA) has organized the revision of the "Quality Management Standards for Medical Device Production" in accordance with the "Regulations on the Supervision and Administration of Medical Devices" (Decree No. 650 of the State Council) and the "Measures for the Supervision and Administration of Medical Device Production" (Decree No. 7 of the CFDA).
- Consult
- National service hotline:
0755-27391220