EU MDR and IVDR registration agency services
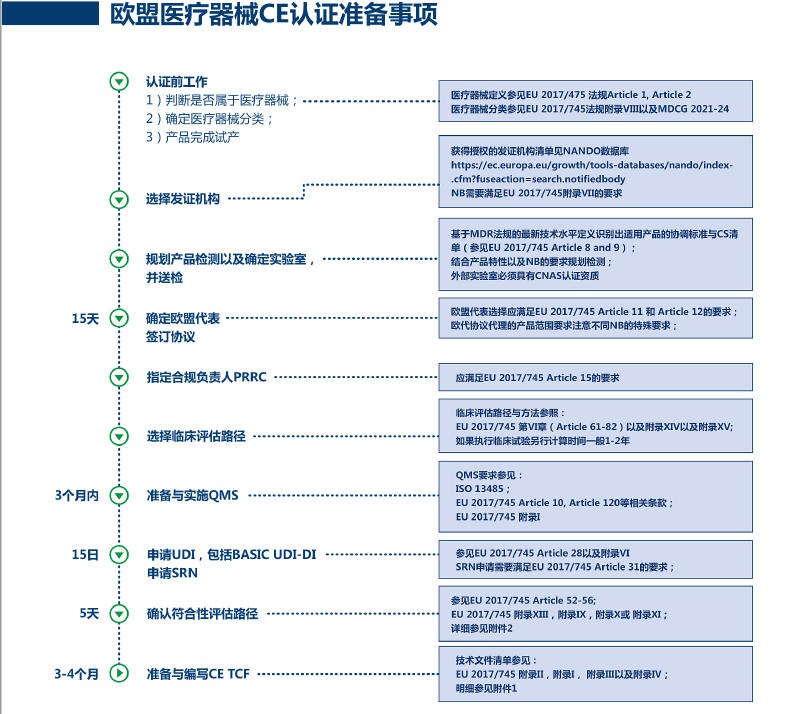
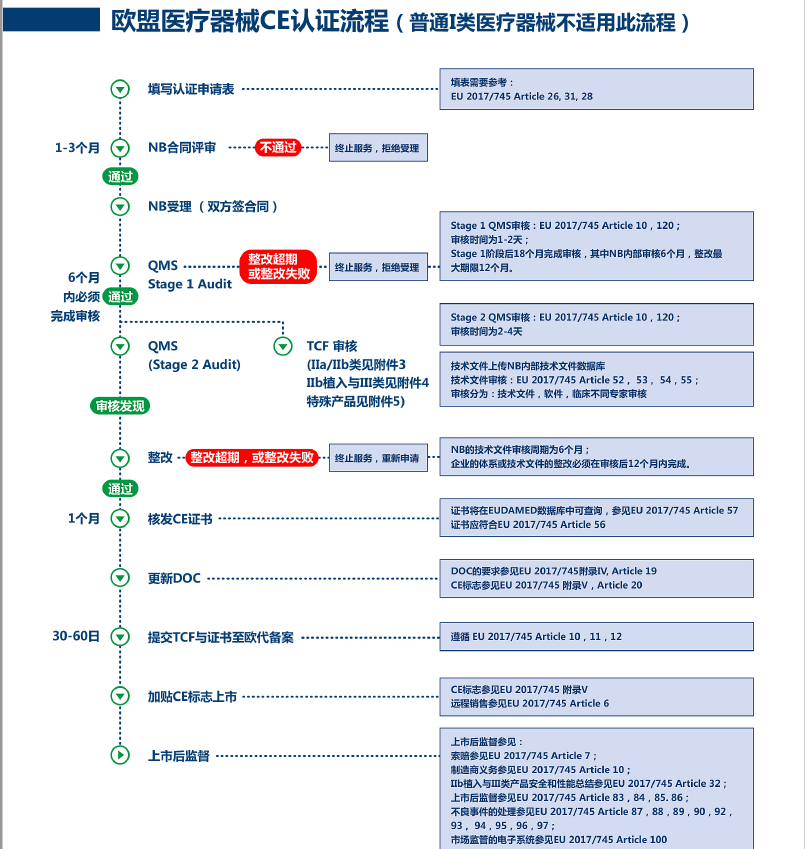
Under the EU CE MDR, products requiring review by a Notified Body (NB) include: Class I (Is, Ir, Im), Class II (Class IIa and Class IIb), and Class III; after the preparation of technical documents for Class I (excluding Is/Ir/Im), they should be submitted to the EU representative for filing. Under the EU CE IVDR, products requiring review by a Notified Body (NB) include: Class A (sterile), Class B, Class C, and Class D; after the preparation of technical documents for ordinary Class A, they should be submitted to the EU representative for filing.
- Consult
- National service hotline:
0755-27391220