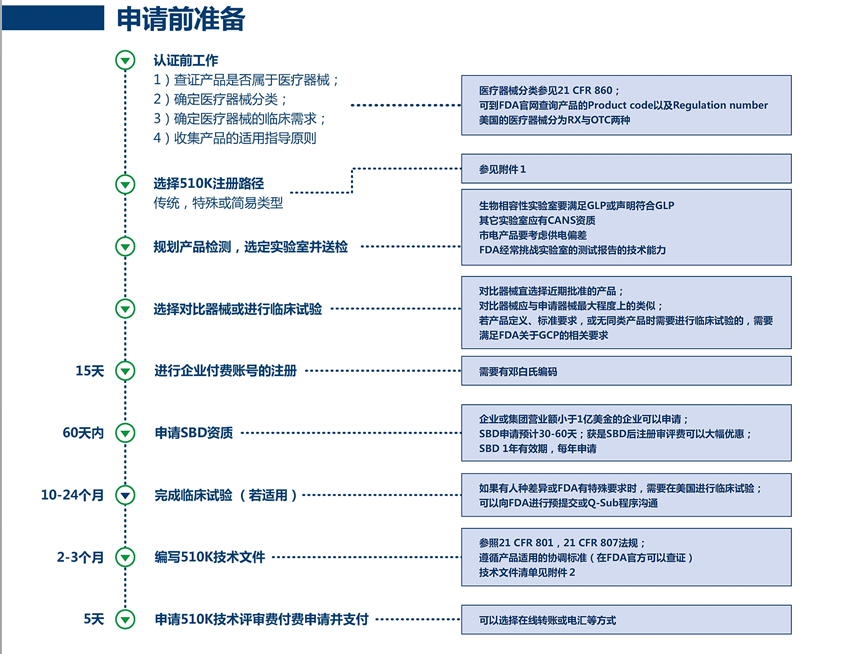
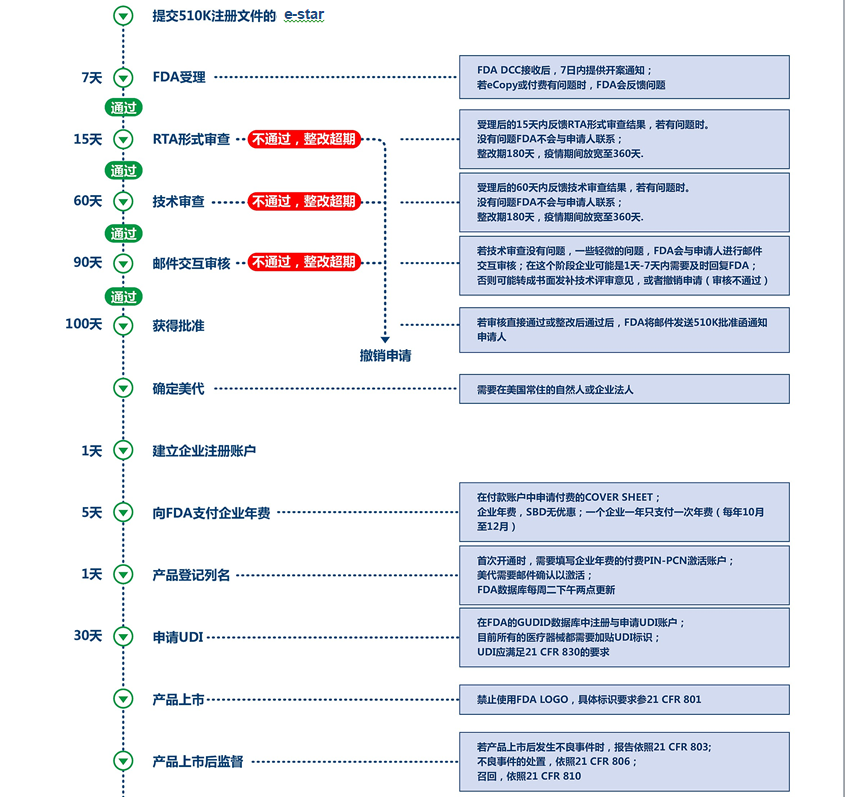
U.S. 510(k) agency registration service
The US FDA 510(k) registration does not apply to: Class I exempted products and Class II exempted products.
- Consult
- National service hotline:
0755-27391220